RECOMMENDATION: BUY
CytRx Corporation (NASDAQ: CYTR)
CYTR Trading at Cash Value
| Market Data: Exchange Symbol CYTR (NASDAQ) Price of Common Stock (1/20/99) $1.81 30-Day Average Trading Volume 55,000 Shares Outstanding 7.7 million 52-Week High/Low $3.56/$0.75 |
CytRx Corporate Information: Address 154 Technology Parkway Norcross, GA 30092 Telephone 770-368-9500 President & CEO Jack J. Luchese Chief Financial Officer Mark W. Reynolds |
Summary Investment Considerations
CYTR is a biotechnology company focused on the development and commercialization of innovative therapeutic products. CYTR's lead product is FLOCOR(, which is in pivotal Phase III clinical trials for the treatment of acute sickle cell vaso-occlusive crisis ("VOC"). CYTR recently announced that its independent Safety and Data Monitoring Board had analyzed data from the first 50 patients of its Phase III trial, and recommended continuation of the trial as planned. We anticipate the trial to be completed by the end of 1999. CYTR is also developing FLOCOR for additional indications, including shock, stroke and acute respiratory disorders. CYTR has a pipeline of technologies in the areas of gene and drug delivery, vaccines and infectious disease. CYTR is trading at a significant discount to biotechnology companies with promising products in Phase III clinical trials, and at roughly the value of cash on hand. We recommend purchase of CYTR for investors tolerant of the risks associated with small-cap equity investments.
I. FLOCOR for Sickle Cell Crisis -- In Pivotal Phase III Trials
- FLOCOR is currently in pivotal Phase III clinical
trials, and we anticipate an interim review of safety and efficacy results
in the second quarter of 1999; CYTR has been granted Orphan Drug Status
for FLOCOR for sickle cell crisis, and received a $400,000 grant from
the FDA to help support the Phase III trial.
- Attractive market: Sickle cell disease affects
from 60,000 to 100,000 individuals in the US, for whom there are no
available therapies to shorten crisis duration or prevent resulting
tissue damage.
- CYTR has signed an agreement with Abbott Laboratories
for the clinical and commercial-scale production of FLOCOR, and for
assistance with regulatory filings; CYTR is actively seeking corporate
partners to assist in marketing efforts internationally
II. Compelling Valuation -- Trading at Cash Value
- We believe the investment case for CYTR, and the
opportunity for FLOCOR, is not widely understood or appreciated by the
investment community; CYTR has low institutional ownership and limited
research sponsorship.
- CYTR currently has approximately $2.00 per share
in cash and cash equivalents; CYTR trades at a significant discount
to valuation levels typical for biotechnology companies with promising
products in pivotal Phase III clinical trials.
III. Pipeline Technologies -- Source of Additional Shareholder Value
- Additional FLOCOR indications: We believe there
are significant opportunities for FLOCOR in the treatment of stroke,
shock and acute respiratory disorders; CYTR is actively seeking corporate
partners to assist in the development and commercialization of FLOCOR
for these and other indications.
- CYTR has promising technology in early stages of
development and is actively seeking corporate partners.
Company Overview
CYTR is a biotechnology company focused on the development and commercialization of innovative therapeutic products. The Company was founded in 1985 and is headquartered outside Atlanta, Georgia. CYTR's current primary focus is on FLOCORTM, a synthetic block copolymer, that is being developed to treat vaso-occlusive disorders, including crisis of sickle cell disease, acute lung injury (ALI)/ acute respiratory distress syndrome (ARDS), stroke and shock. FLOCOR is currently in a pivotal Phase III trial in the US to treat sickle cell crisis.CYTR has, in the past, created operating subsidiaries to develop technologies that were considered non-core. In 1998, CYTR divested of two of these subsidiaries (Vetlife and Proceutics), and is currently looking for partners or buyers of its remaining subsidiary called Vaxcel, Inc. These corporate divestiture activities provided cash to CYTR in 1998. See CYTR's SEC filings for a complete description of these activities.
Sickle Cell Diseases -- Background
Hemoglobin is the vehicle by which oxygen is transported from the lungs and delivered throughout the body. Hemoglobin resides inside red blood cells. Normal red blood cells are doughnut shaped and can easily maneuver throughout the body. Sickle cell diseases occur as a result of genetic mutations to certain parts of the hemoglobin molecule that result in the sickling of red blood cells after oxygen has been released. (See figures below.) The cells become elongated, rigid and sticky and cause blockages in the bloodstream that result in many complications for patients, including acute pain episodes, strokes, increased infections, leg ulcers, bone damage, gallstones, lung blockages, kidney damage, blood blockage in the spleen or liver, eye damage, anemia, delayed growth and sepsis.
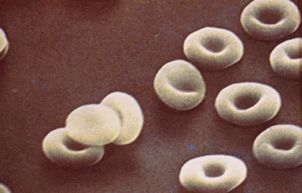
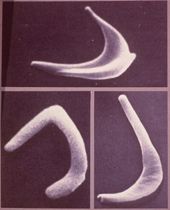
Normal hemoglobin is called type A, sickled hemoglobin is called type S, and other mutations have the designations C and E. Fetal hemoglobin, type F, has been shown to have properties that protect red blood cells from sickling and can help reduce complications. There are three sickle cell syndromes: sickle cell anemia, (HbSS), hemoglobin SC (HbSC) and hemoglobin S beta thalassemia (HbSBeta-thal). Sickle cell anemia is the most common variant and, while it predominantly affects people of African American descent, it also affects Arabs, Greeks, Italians, Latin Americans and Native Americans. The median survival of HbSS patients is about 45 years, primarily due to repeated trauma to internal organs from ischemia (oxygen deprivation) and inflammatory responses.It is estimated that anywhere from 60,000 to 100,000 people in the US have sickle cell disease, or roughly 1 in 400 African Americans. Approximately 1 in 10 African Americans are "carriers," that is, they carry one copy of the S gene, but do not have the disease. It is also estimated that complication from sickle cell diseases result in healthcare expenditures of about $1.0 to 1.5 billion annually in the US.
The most common problem sickle cell patients face is episodic pain (also referred to as vaso-occlusive crisis, or VOC). These episodes can last anywhere from 5 minutes to days to weeks and can vary significantly in their severity. While the exact cause of the pain is unknown, it is believed to be caused by inflammatory response to bone marrow necrosis, ischemic muscle and ischemic bowel, resulting from blood flow blockage. It's been suggested that precipitating factors include infection, dehydration, increased anemia, acidosis from any cause, emotional stress, extreme temperature exposure or ingestion of drugs or alcohol.
Current Treatments: Until recently, the only treatments for sickle cell disease and the resulting pain episodes were palliative -- administration of analgesics, including narcotics and NSAIDs, re-hydration, bed rest, and the treatment of underlying infection and other precipitants. Physicians recommend that certain preventive measures be taken to minimize pain episodes. These include the administration of folate, to help stimulate red blood cell production, keeping fully hydrated (drinking 8-10 glasses of water or fluid per day), keeping immunizations up to date, patients and parent awareness of fevers and signs of infection, the avoidance of extreme temperature changes, appropriate dress for the weather, and understanding physical limitations in sports and outdoor activities.
Over the last few years, blood transfusion has been shown to be effective for prophylaxis of acute stroke and for treatment of acute chest syndrome in sickle cell patients, especially children. Although the treatment is beneficial, it is associated with transfusion reactions and exposes patients to blood-borne pathogens such as HIV and hepatitis virus. In addition, patients develop antibodies after multiple transfusions, making future transfusion therapy difficult.
In March 1998, the FDA approved hydroxyurea (Hydrea or Droxia®) for treatment of adults (over 18) who suffer from sickle cell anemia and have had at least three painful crises during the previous year. Hydrea is a cytotoxic chemotherapeutic agent that has been shown to enhance HbF production, thus aiding in interfering with the polymerization of HbS in solution and with the sickling of red blood cells. Treatment with Hydrea was found to reduce pain episodes, reduce the need for blood transfusions, and reduce hospital admissions by 50%. It is estimated that roughly one third of sickle cell patients in the US are currently taking hydroxyurea. While it is by no means a cure, it appears that Hydrea does help control the symptoms of the disease. However, almost all the patients who received Hydrea in clinical trials needed to have their medication stopped for a time to allow their blood count to return to acceptable levels. In addition, there is concern that prolonged use of Hydrea may result in the increased incidence of cancer. Hydroxyurea, marketed as Droxia by Bristol-Myers Squibb is also approved by the FDA to treat certain types of leukemia and other cancers.
FLOCORTM
CytRx's most advanced clinical drug candidate is FLOCORTM. FLOCOR is a highly purified form of the surfactant poloxamer 188, a synthetic block copolymer, and is being developed as an intravenous (IV) formulation to treat vaso-occlusive disorders, including crisis of sickle cell disease, acute lung injury (ALI)/ acute respiratory distress syndrome (ARDS), stroke and shock.Normally, blood cells flows very smoothly through the circulatory system, passing each other and blood vessel walls without incident, due to non-adhesive surfaces. When cells are damaged or certain disease is present, exposed areas cause cells to become sticky and can impede blood flow and delivery of oxygen, creating vaso-occlusive crises. As discussed above, sickle cell crisis is one example of this.
Poloxamer 188 has been commercially available since the 1950s and has been used as a surfactant/emulsifying agent and as a food additive and excipient in pharmaceutical products. In the mid-1980s, CytRx discovered and patented a formulation of commercial grade poloxamer 188, which it called RheothRx, for use as an intravenous therapeutic for obstructive vascular disorders. Following successful Phase I studies, CytRx licensed worldwide rights to Burroughs Wellcome (BW) in 1990. BW, now Glaxo Wellcome, conducted Phase II trials in both sickle cell crisis and acute myocardial infarction (AMI), and found the RheothRx formulation to be well tolerated and efficacious. However, in a larger AMI study, at the effective dose, a small percentage of patients began experiencing transient elevations in creatinine, indicating decreased kidney function. In 1996, Glaxo Wellcome returned RheothRx to CytRx.
Upon further examination of poloxamer 188, certain impurities were found that were shown to be responsible for the elevated creatinine levels. At that time, CytRx developed a process for removing the impurities associated with kidney dysfunction, without altering the beneficial properties of the compound. The purified compound, FLOCOR, demonstrated no elevation of creatinine following 48 hours of continuous infusion at doses up to 33% higher than those previously discontinued.
FLOCOR has been shown to improve blood flow by binding to any exposed area of a cell or molecule that is even slightly more adhesive than the surrounding materials thus allowing cells to slip by each other and by blood vessel walls. FLOCOR may also reduce secondary clotting, reduce inflammation and bind to harmful molecules that could injure the respiratory system.
CytRx was issued a composition of matter patent on FLOCOR in the US in June 1997, has received a notice of issue for Europe, and has a substantial portfolio of issued US (and corresponding foreign) patents on various therapeutic uses of poloxamer 188. More importantly, in meetings with the FDA, CytRx has been notified that the existing pharmacology, toxicology and human safety data for commercial grade poloxamer 188 could be used to support an NDA submission for FLOCOR. In April 1998, CytRx announced an agreement with Abbott Laboratories for commercial scale manufacturing of FLOCOR. Under the agreement, Abbott will sterile fill FLOCOR for CytRx as well as manage their portion of the chemistry manufacturing and controls (CMC) section of the planned NDA filing.
FLOCORTM for Sickle Cell Crisis
CytRx is currently involved in a pivotal Phase III study of FLOCOR for the treatment of painful vascular occlusive crisis of sickle cell disease. The 45 to 50-center trial will involve 224 patients, ages 10 to 65, and, we estimate, should be completed by the end of 1999. FLOCOR is being delivered by IV infusion over a 48-hour period.In a Phase II trial, FLOCOR demonstrated positive results, reducing the duration of crisis by 16 to 45%, reducing the need for pain medication by 2.8- to 4.3-fold, reducing pain intensity by 40 to 45% and reducing hospital stay by 1 to 2 days. For the ongoing Phase III studies, the primary endpoints are reductions in the duration of crisis, the duration and intensity of pain, total analgesic use and duration of hospitalization. CytRx recently announced a favorable review from an independent Safety and Data Monitoring Board following examination of data from the first 50 enrolled patients in the trial (as of January 12, CytRx had enrolled 86 patients).
If all goes well, we are anticipating an NDA filing on FLOCOR for sickle cell crisis in mid-2000. Based on the fact that FLOCOR has been designated as an Orphan Drug, and is being proposed to treat a severely debilitating disorder, we feel it is likely to receive an expedited review at the FDA. The Orphan Drug Products Division of the FDA has already granted CYTR an unusual $400,000 grant to help complete the Phase III trial. We are therefore anticipating final approval of FLOCOR by the end of 2000, with an early 2001 launch.
CYTR has already planned follow-on studies on the recurrent use of FLOCOR in sickle cell patients.
Marketing Issues:Due to the fact that the majority of sickle cell patients live in urban settings, the marketing of FLOCOR in the US should require a relatively small salesforce. According to industry statistics, roughly half of the US sickle cell patients reside in the top 16 metropolitan areas, with 40% residing in the top 10. In addition, FLOCOR will most likely be marketed directly to hematologists in sickle cell centers. CYTR is therefore committed to marketing FLOCOR itself in the US and partnering marketing rights outside the US. We have built the cost of a 12-person US sales and marketing force into our earnings model.
The company has had 2 independent consultant groups assess the cost/benefit of FLOCOR use. Based on the early clinical results, each of the groups concluded that $2,000 per hospital stay (5 vials of FLOCOR at $400 per vial) is a reasonable price for the product. We have therefore built these pricing assumptions into our sales model (see Financial Information and Valuation Discussion).
Potential Competition/Other Treatments in Development
Due to the severity of sickle cell disease and the associated mortality rates, many different treatments are being explored. However, we believe that many of these potential treatments are still a long way off. In addition, the treatment of sickle cell disease is a new market, and therefore we believe there will be sufficient opportunity for multiple products to successfully enter and prosper in this market.Successful bone marrow transplantation can cure sickle cell anemia, however, carries with it a 1-in-10 mortality rate. And, since candidates for transplantation are usually the most severely afflicted, they are often less capable of handling an arduous procedure. It is also recommended that only related donors be used and thus the supply is limited. However, a national collaborative study of transplantation in pediatric patients is underway with strictly defined eligibility criteria. Umbilical cord blood, an alternative to bone marrow, may prove to be useful in stem cell transplantation; the supply of "banked" cord blood continues to grow in the US and cord blood compatibility requirements are less strict.
Cypros Pharmaceutical Corporation announced at the end of 1998 that it had begun enrolling patients in a Phase III trial in the US on CordoxTM in sickle cell anemia crisis patients. Cordox, formerly known as CPC-111, is a natural sugar phosphate that provides metabolic energy to blood-deprived tissues. Also in December, Cypros announced that the FDA has granted expedited development status to the drug. In a Phase II study completed in 1997, Cypros showed that Cordox significantly reduced pain during crisis in sickle cell anemia patients. Cypros is developing Cordox for several ischemic indications including bypass surgery, heart transplant and sickle cell crisis.
Another promising development has come out of Duke University Medical Center, where a group of researchers has used gene therapy to correct the defect in human blood cells that causes sickle cell anemia. In the June 5, 1998 issue of Science, the group published an article demonstrating that, rather than correct the faulty DNA, ribozymes can be used to specifically correct the defective RNA produced from the DNA, and from which the mutated hemoglobin is made. In all the precursor red blood cells used in the experiments, the ribozymes were shown to successfully splice in the correct RNA sequence. The researchers believe that the most likely candidates for ribozyme therapy would be severe sickle cell patients who have developed antibodies to components of transfused blood. It may be possible to correct the sickle cell trait in the patients' own cells, and then re-administer them to the patient. Animal testing was scheduled to begin in late 1998. Although promising, we believe commercial gene therapy is still 5-10 years down the road.
Positive results have also been achieved using Nitric Oxide (NO)‹note this is not nitrous oxide, or "laughing gas," used as a light anesthetic. NO has been used to treat certain lung ailments and has been shown to cause smooth muscle in blood vessel walls to relax and to dilate the entire vessel. In the laboratories at the University of Chicago, NO, even at the lowest concentrations, was shown to slow red blood cell sickling and even promoted the unsickling of sickled cells. In separate studies at Massachusetts General Hospital (MGH) and other Boston hospitals, it was shown that, by causing sickle hemoglobin molecules to bind oxygen with greater affinity, NO reduced the sickling in both laboratory studies and in several volunteer patients who breathed low concentrations of NO.
FLOCORTM for Other Indications
CytRx is also developing FLOCOR for other vaso-occlusive disorders, the most advanced of which is a Phase I program to treat acute chest syndrome (ACS), an acute respiratory distress syndrome (ARDS) -- like complication occurring in sickle cell patients. ACS occurs in approximately 10-20% of hospitalized sickle cell patients.The Company is also investigating FLOCOR's use in a general population of Acute Lung Injury (ALI)/ARDS patients in a small pilot trial. ARDS is characterized by the inability to deliver oxygen to the circulatory system and is usually brought on by severe chest trauma such as smoke inhalation, accidents, chemical damage or infection. ALI/ARDS has a high mortality rate and high related healthcare costs. CYTR is expecting to begin a pilot Phase II study in ALI/ARDS in the second quarter of 1999.
Due to FLOCOR's inherent properties, it would appear that the compound could be of use in stroke, where blood flow is restricted to the brain, in circulatory shock, where low blood pressure can result in organs receiving inadequate oxygen, and in heart attack, where partial or complete blockage of arteries causes severe pain and damage to the heart muscles. For these larger indications, CYTR plans to license these indications to FLOCOR and we have included licensing revenues over the next several years in our earnings model.
Other Pipeline Technologies
While the development emphasis at CYTR is clearly focused on FLOCOR for sickle cell disease and other indications, CYTR has additional pipeline technologies that we believe will be a source of additional shareholder value. CYTR is actively seeking corporate partnering arrangements for these additional technologies.Vaccine Adjuvants (Phase I): CYTR has patented the use of a series of poloxamers as vaccine delivery systems, or vaccine adjuvants, to enhance the effectiveness and/or convenience of currently marketed or new vaccines. These systems have potential in both injectable, oral and mucosal vaccines. CYTR has licensed the rights for human therapeutic use of these compounds to its subsidiary, Vaxcel, of which CYTR owns 87.5% of the outstanding common shares. CYTR is actively seeking a corporate partner for these technologies.
Anti-Microbial, CRL-1072 (Pre-clinical): CRL-1072 is a purified poloxamer that has exhibited, in animal models, high potent activity against a wide range of infectious agents. CRL-1072 has shown activity in animal models of fatal Mycobacterium tuberculosis, Mycobacterium avium and Toxoplasmosis infection, and was also active against drug resistant isolates of M. tuberculosis. The compound has also been shown to reduce viral load and viral reactivation in models of chronic hepatitis B and herpes virus I and II. CYTR has worldwide composition of matter patent protection on this compound.
P-Glycoprotein Inhibitor Program (Research): CYTR has licensed a series of novel, non-toxic inhibitors of the drug efflux pump P-glycoprotein, from Rush Presbyterian/St. Luke's Medical Center. These compounds, which have an excellent safety profile, could be used to enhance bioavailability of poorly absorbed oral drugs. CYTR's strategy is to out-license this technology.
CYTR has other additional technologies that it is actively seeking to partner or out-license. For example, CYTR has patented the use of certain poloxamers for delivery of genes for gene therapy applications. Poloxamers may be as effective as cationic lipids, a focus of many different gene therapy companies, but are not metabolized and may be less toxic. CYTR is also working on an animal feed additive that has been shown to improve the rate of weight gain and feed efficiency in studies in poultry and swine. This technology is patented as well.
Other CYTR Businesses
CYTR manufactures, markets and distributes Titermax®, an adjuvant used to produce cell-mediated and humoral responses in research animals. The keys to Titermax lie in its immunostimulatory activity and the formation of stable water-in-oil emulsions. Titermax aids in the antigen's presentation to the immune system without the toxic effects of other research adjuvants. We estimate that sales of Titermax should remain stable at $400,000 to $500,000 per year. (See Model.)CYTR has also formed Spectrum Recruitment Research, a small group of human resource professionals who, in addition to performing services to the Company, provide third-party recruiting services. We estimate that revenues from Spectrum should remain stable at about $500,000 per year.
Revenues from Spectrum and Titermax are included on the other revenue line in our earnings model. We consider these businesses to be non-core, stable and profitable, and are not a drain on the Company's cash resources.
Management
Jack J. Luchese joined CYTR in March of 1989, is currently President and CEO. Prior to joining CYTR, Mr. Luchese held positions at various companies in the pharmaceutical industry including a fifteen-year relationship with Johnson & Johnson Company. Immediately prior to joining the Company, Mr. Luchese served as Vice President and General Manager of Armour Pharmaceutical Corporation, a wholly owned subsidiary of Rhone-Poulenc Rorer. Mr. Luchese received his master's degree in business administration from Fairleigh Dickinson University and his bachelor's degree in business from Montclair University.R. Martin Emanuele, Ph.D. joined CYTR in 1988 and is currently Vice President, Research and Business Development. Prior to joining CYTR, Dr. Emanuele was a clinical research scientist at DuPont Critical Care and a visiting scientist at Institute Choay. Dr. Emanuele received his Ph.D. in pharmacology and experimental therapeutics from Loyola University of Chicago. He also earned a master's degree in biology from Northern Illinois University and a bachelor's degree in biology from Colorado State University.
William B. Fleck joined CYTR in 1993, and currently is Vice President, Human Resources. Prior to joining CYTR, Mr. Fleck held senior human resource management positions at various companies, most recently serving as Director, Human Resources and Training for Columbia/HCA. Mr. Fleck earned his bachelor's and master's degree in Personnel Management from Florida State University.
J. Michael Grindel, Ph.D. joined CYTR in October of 1997 and currently serves as Vice President, Drug Development. Prior to joining CYTR, Dr. Grindel held various management positions at pharmaceutical firms, most recently serving as Vice President, Preclinical Development for Hybridon, Inc. Dr. Grindel received his Ph.D. in medicinal chemistry from University of Kansas and his bachelor of science degree from St. Benedict's College (Kansas).
Mark W. Reynolds joined CYTR in 1988 and currently is Chief Financial Officer and Corporate Secretary. Prior to joining CYTR, Mr. Reynolds was a certified public accountant with Arthur Andersen LLP. Mr. Reynolds holds BBA and MACC degrees from the University of Georgia.
Financial Information and Valuation Discussion
With input from CYTR management, and based upon market information regarding the potential market for an effective therapy for sickle cell disease, we have generated financial projections for CYTR (see below). The primary focus of the model, and the investment case, are the potential domestic and international markets for FLOCOR for sickle cell disease. We have made the assumption that CYTR retains marketing rights to FLOCOR for the sickle cell indication in the US, and forms alliances with international marketing partners for the Brazil and South America, Caribbean and Latin American, and European markets. We believe that our assumptions -- size of markets, pricing for FLOCOR, ability to penetrate international markets, etc. -- are achievable and are sufficiently conservative. We believe our margin assumptions are also achievable.In an effort to be conservative, we have assumed only modest contributions in the out-years for other FLOCOR indications -- specifically, the potentially large market opportunities in the areas of stroke, shock and acute lung injury/acute respiratory distress. We have also not included contributions from other potential technologies such as the CRL-1072 antiviral, the vaccine adjuvants program or the CRL 1095 drug delivery technology. We believe these technologies are very interesting, and a potential source of shareholder value, but unlike FLOCOR for sickle cell, are in very early stages of development.
Potential Upside -- Market Expansion: Currently, the only available treatments for sickle cell patients in vaso-occlusive crisis are intravenous painkillers and hydration. Clinicians frequently tell sufferers not to come to the hospital until the pain becomes unbearable, as there is little that can be done for them. As sufferers typically can tell when a crisis is beginning, an effective treatment like FLOCOR may encourage sufferers to seek treatment earlier and at higher rates than currently observed. We have made a conservative assumption on market expansion, and believe the actual expansion could occur at higher rates.
Valuation: We have provided a variety of different valuation methodologies, including a discounted cash flow model, terminal P/E multiples and terminal operating income multiples. We believe we were sufficiently (if not excessively) punitive on our discount rates, especially considering the Company is in Phase III clinical trials with FLOCOR for sickle cell following very positive Phase II data. With respect to asset values, the Company, as of a corporate announcement on December 9, 1998, had approximately $16 million (or over $2.00 per share) of cash and cash equivalents. Regardless of the valuation methodology, CYTR's share price appears undervalued at its current level and we believe current fair value to be in the range of $8 to $10 per share. Based upon corporate milestones expected over the next 12 months, specifically interim efficacy data on the Phase III trial, we believe an appropriate 12-month price target of $5 to $7 is achievable.
Earnings Model and Valuation Analyses: See Below
Summary Balance Sheet ($000s)
Cash & equivalents
Receivables
Total Assets
Long term debt
Shareholders' equity12/31/97
$5,895
1,917
24,906
0
19,2489/30/98
$13,372
4,469(1)
22,168
0
20,545
Note 1: A $4.0 million note receivable was received in the fourth quarter of 1998.
Risk Considerations
This section of the document is provided to remind potential investors to undertake a prudent level of due diligence prior to making an investment in the securities of CytRx Corporation. For a complete description of risks and uncertainties to CYTR's business, see the "Risk Factors" section in CYTR's SEC filings, which can be accessed directly from the SEC Edgar filings at www.SEC.gov on the internet. Other potential risks include:
- Market risk: Like many small-cap and micro-cap
stocks, CYTR share price is trading near its 52-week low. Investors
should consider technical risks common to many small-cap or micro-cap
stock investments, including liquidity levels, small float, risk of
dilution, dependence upon key personnel, dependence upon single products
or technologies, and the strength of competitors that may be larger,
better capitalized and hold dominant market positions.
- Business risk: CYTR has limited experience in
the manufacturing, marketing, and the distribution of pharmaceutical
products. Many of its products are in the early stages of development.
Additionally, CYTR intends to license rights to its products to other
companies. There can be no assurance that these licensing agreements
will be completed, or that the market will accept any products under
development.
- Regulatory risk: There is no guarantee that CYTR's
products will be approved by the US FDA or international regulatory
bodies for marketing in the US or abroad.
- Competitive risk: The pharmaceutical industry
is extremely competitive, in particular because of its large market
potential. Many companies are developing products for sickle cell anemia
and other therapeutic indications targeted by CYTR.
For Additional Information
Contact SmallCaps Online LLC -- 212-554-4158Sources for Additional Information
The following are website addresses offering related information, and links to other sources of information.www.cytrx.com
www.SmallCapsOnline.com
www.FDA.gov
www.WHO.int
www.curtis1/sickle_cell_anemia.html
www.sicklecellsociety.org
www.emory.edu/PEDS/SICKLE/
www.SEC.gov
CytRx's corporate website
SmallCaps Online's site for
company information and research
US Food and Drug Administration homepage
World Health Organization homepage
Sickle Cell Anemia Disease Links
Sickle Cell Society homepage
Sickle Cell Disease site
US Securities and Exchange Commission,
with links to EDGAR filings
The information in this report has been obtained from sources that we believe to be reliable, but we do not guarantee its accuracy or completeness. Neither the information nor any opinion expressed constitutes a solicitation by SmallCaps Online LLC for the purchase or sale of any securities. SmallCaps Online LLC may have performed investment banking, consulting or other services for or may solicit investment banking, consulting or other business from, any company mentioned in this report. SmallCaps Online LLC or persons associated with SmallCaps Online LLC may at anytime be long or short any of the securities referred to herein and may make purchases or sales thereof while this report is in circulation or posted on the SmallCaps Online LLC website at www.SmallCapsOnline.com. This material, or any portion thereof, may not be reproduced without prior permission from SmallCaps Online LLC. SmallCaps Online LLC is not responsible for the contents of this document that is intended for electronic transmission and could be thus subjected to tampering or alteration. Copyright © 1999 by SmallCaps Online LLC. All rights reserved.