 |
|
Title Page | Introduction | Principles
of Intraperitoneal Chemotherapy | Current Indications for
Cytoreductive Surgery and Intraperitoneal Chemotherapy
Heated
Intraoperative Intraperitoneal Chemotherapy by the Coliseum
Technique
Immediate
Postoperative Abdominal Lavage in Preparation for Early
Postoperative Intraperitoneal 5-Fluorouracil
Early
Postoperative Intraperitoneal Chemotherapy for Adenocarcinoma | Induction
Intraperitoneal Chemotherapy for Debilitating Ascites
Cytoreductive
Surgery for Peritoneal Surgacy Malignancy - Pertitonectomy
Procedures | Results of Treatment of
Peritoneal Surface Malignancy
Conclusions | References
| VII. | CYTOREDUCTIVE SURGERY FOR PERITONEAL
SURFACE MALIGNANCY - PERITONECTOMY PROCEDURES |
Not all six of these peritonectomy procedures will be required in
all patients. The peritoneal surfaces are stripped of tumor only
where there is visible disease. The surgeon's goal with the
cytoreduction is to remove as much tumor as is possible (9). The
smaller the cancer volume that remains for chemotherapy
treatments, the better the results will be.
 |
|
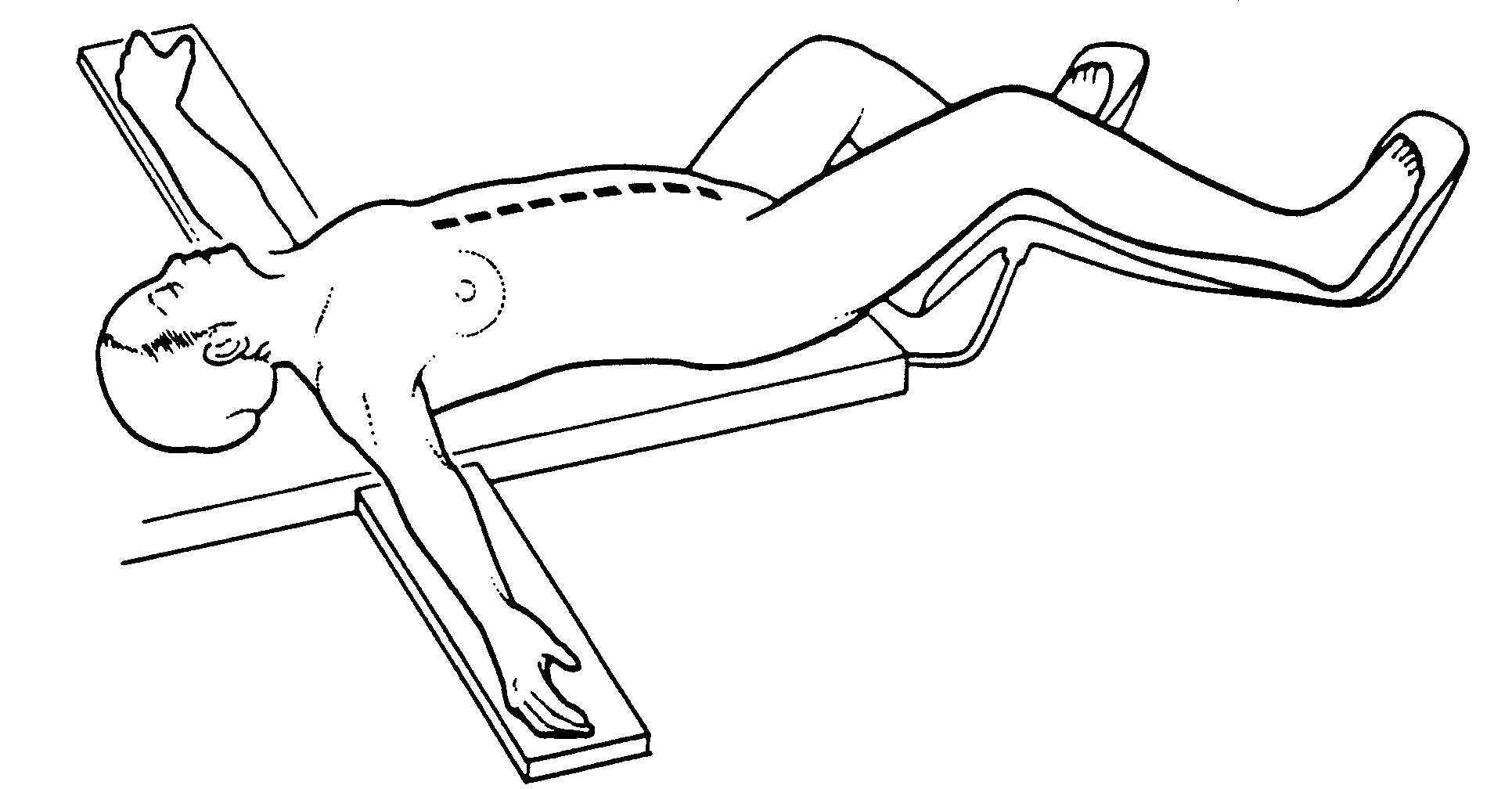
Position and incision for peritonectomy procedures. From
Sugarbaker PH: Peritonectomy procedures. Ann Surg 221:29-42, 1995
(with permission).
Position and incision
The patient is placed in a supine position with the gluteal
folds advanced to the break in the operating table in order to
provide full access to the perineum during the surgical procedure
(Figure 10). This modified lithotomy position is achieved
with the legs extended in St. Mark's leg holders (AMSCO, Erie,
PA). The weight of the legs must be directed to the bottom of the
feet by positioning the footrests so that minimal weight is borne
by the calf muscle.
All surfaces of the St. Mark's stirrups are protected by egg
crate foam padding. The thighs and legs are surrounded by
alternating pressure devices (SCB Compression Boots, Kendall Co.,
Boston, MA). These should be operative prior to the induction of
anesthesia for maximal protection against venothrombosis. A
hyperthermia blanket is placed over the chest. arms and head of
the patient (Bair Hugger Upper Body Cover, Augustine Medical,
Eden Prairie, MN), and also beneath the torso (Cincinnati
Sub-Zero, Cincinnati, OH).
Abdominal skin preparation is from mid-chest to mid-thigh. The
external genitalia are prepared in the male, and in the female, a
vaginal preparation is also used. The Foley catheter is inserted
after the skin preparation so that this catheter can be accessed
during the surgical procedure. A large bore silastic nasogastric
tube is placed within the stomach (Argyle Silastic Salem Sump
Tube, Sherwood Medical, St. Louis, MO).
Abdominal exposure, greater omentectomy, and splenectomy
The abdomen is opened from xiphoid to pubis (Figure 11).
The xiphoid is excised using a rongeur. Generous abdominal
exposure is achieved through the use of a Thompson Self-Retaining
Retractor (Thompson Surgical Instruments, Inc., Traverse City,
Ml). The standard tool used to dissect tumor on peritone4
surfaces from the normal tissues is a ball-tip electrosurgical
hand piece (Valleylab, Boulder, CO). The ball-tip hand piece is
placed at the interface of tumor and normal tissues. The focal
point for further dissection is placed on strong traction. The
electrosurgical generator is used on pure cut at high voltage
(Birtcher Electrosurgical, Englewood, CO). The 3mm ball-tip
electrode is used for dissecting on visceral surfaces including
stomach, small bowel, and colon. When more generalized tumor
destruction is required, for example on the liver surface, the
ball-tip will cause more rapid tumor destruction if used in the
hockey stick configuration.
Using ball-tip electrosurgery on pure cut creates a large volume
of plume because of the carbonization and electroevaporation of
tissue. In order to maintain visualization of the operative field
and to preserve a smoke-free atmosphere in the operating theater,
a smoke filtration unit is utilized (Stackhouse, Inc., El
Segundo, CA). The vacuum tip is maintained two to three inches
from the field of dissection whenever electrosurgery is in use.
In order to free the mid-abdomen of a large volume of tumor, a
complete greater omentectomy is performed. The greater omentum is
elevated and then separated from the transverse colon using
ball-tip electrosurgery. This dissection continues beneath the
peritoneum that covers the transverse mesocolon, in order to
expose the anterior surface of the pancreas. All branches of the
gastroepiploic vessels on the greater curvature of the stomach
are clamped, ligated, and divided. Also, the short gastric
vessels are transected. With traction on the spleen, the anterior
fascia of the pancreas is elevated from the gland Using ball-tip
electrosurgery. This freely exposes the splenic artery and vein
at the tail of the pancreas. These vessels are ligated in
continuity and proximally suture ligated. The specimen of greater
omentum and spleen is often free at this point for submission to
pathology. In some patients, the left upper quadrant
peritonectomy must be completed before the specimen is released.
 |
|
Abdominal exposure, greater omentectomy, and splenectomy. From
Sugarbaker PH: Peritonectomy procedures. Ann Surg
221:29-42, 1995.
Left upper quadrant peritonectomy
In order to begin exposure of the left upper quadrant, the
portion of the peritoneum, which constitutes the edge of the
abdominal incision, is stripped away from the posterior rectus
sheath (Figure 12). To secure this peritoneal layer, Kelly
clamps are placed on it at 10 cm intervals. This allows traction
to be achieved on the tumor specimen throughout the left upper
quadrant.
The left upper quadrant peritonectomy involves the stripping of
all tumor tissue from beneath the left hemidiaphragm to expose
diaphragmatic muscle, left adrenal gland, distal portion of the
pancreas, and the cephalad one-half of the perirenal fat. In
order to achieve full exposure of the left upper quadrant, the
splenic flexure is released from the left abdominal gutter and
moved medially by dividing tissue along Toldt's line.
Stripping of tumor tissue from beneath the left hemidiaphragm is
accomplished with ball-tip electrosurgery and not by blunt
dissection. Numerous blood vessels between the diaphragm muscle
and its peritoneal surface must be electrocoagulated before their
transection or unnecessary bleeding will occur. Tissues are
transected using ball-tip electrosurgery on pure cut, but all
blood vessels are electrocoagulated prior to their division.
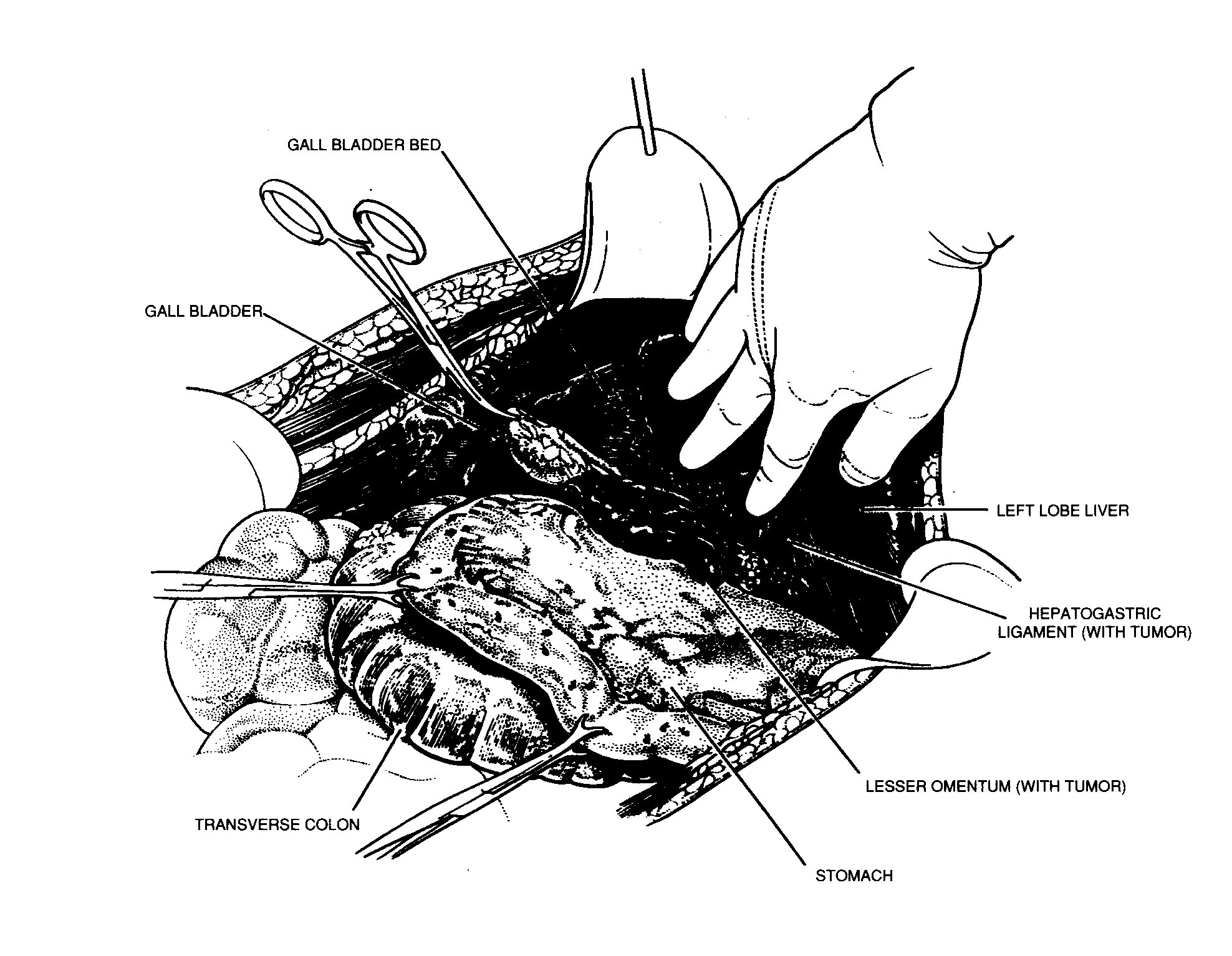 |
FIGURE 12 |
Peritoneal stripping from beneath the left hemidiaphragm.
From Sugarbaker PH: Peritonectomy procedures. Ann Surg
221:29-42, 1995.
Left upper quadrant peritonectomy completed
When the left upper quadrant peritonectomy is completed the
stomach may be reflected medially. Numerous ligated branches of
the gastroepiploic vessels are evident. The left adrenal gland,
pancreas, and left Gerota's fascia are completely exposed, as is
the anterior peritoneal surface of the transverse mesocolon. With
all the upper abdominal peritonectomy procedures, the surgeon
must carefully avoid the major branches of the left gastric
artery and coronary vein in order to preserve the sole remaining
vascular supply to the stomach (Figure 13).
 |
|
Left upper quadrant peritonectomy completed. From Sugarbaker PH:
Peritonectomy procedures. Ann Surg 221:29-42, 1995.
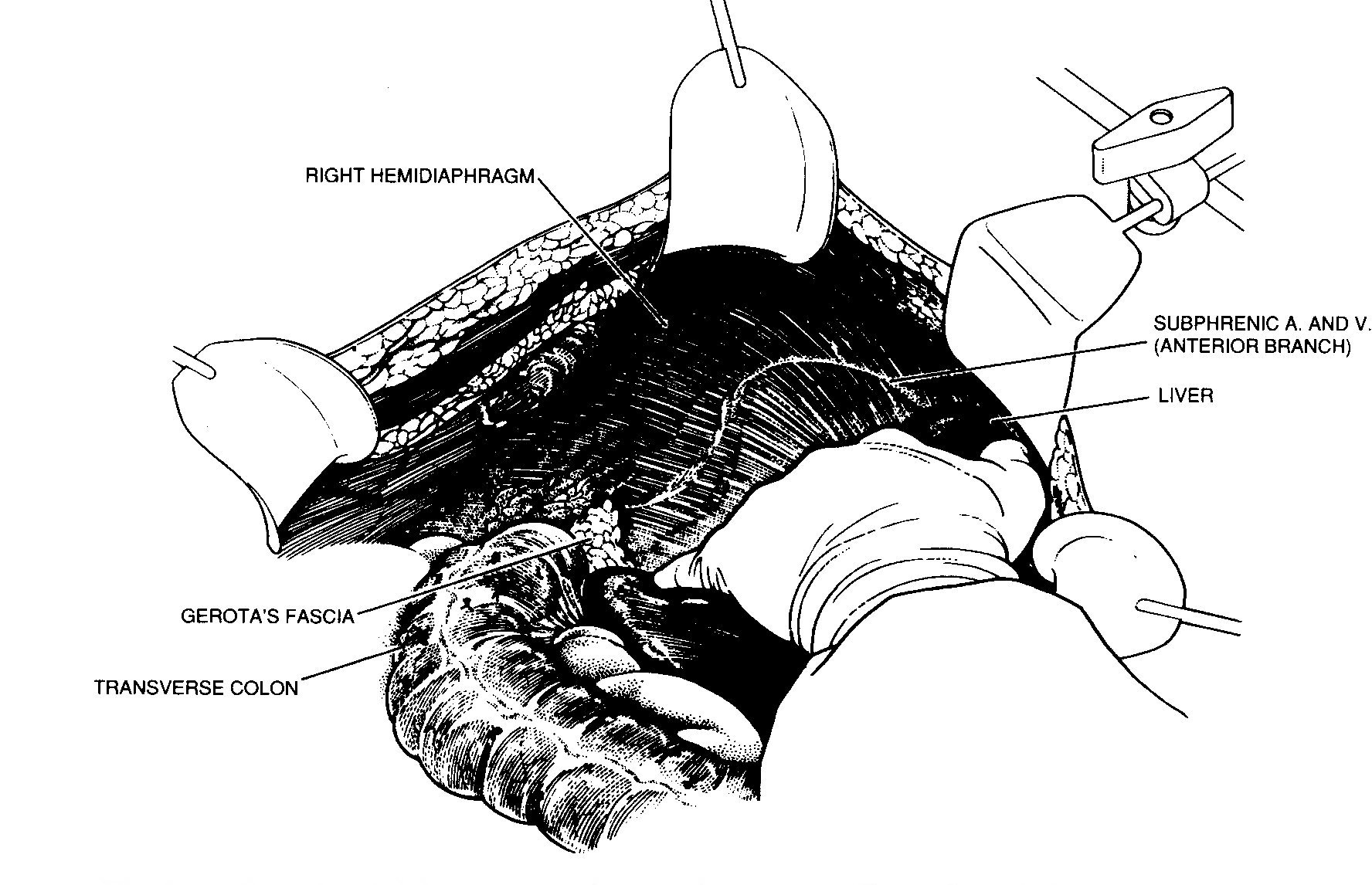
Right upper quadrant peritonectomy
Peritoneum is stripped away from the right posterior rectus
sheath to begin the peritonectomy in the right upper quadrant of
the abdomen (Figure 14). Kelly clamps are placed on the
specimen and strong traction is used to elevate the hemidiaphragm
into the operative field. Again, ball-tip electrosurgery on pure
cut is used to divide tissue planes. Coagulation current is used
to seal blood vessels as they are encountered.
 |
|
Peritoneal stripping from beneath the right hemidiaphragm. From
Sugarbaker PH: Peritonectomy procedures. Ann Surg
221:29-42, 1995.
Dissection beneath tumor through Glisson's capsule
The stripping of tumor from the muscular surface of the
diaphragm continues until the bare area of the liver is
encountered (Figure 15). At this point, tumor on the
anterior surface of the liver is electroevaporated until the
liver surface is visualized. With electrosurgical dissection, one
lifts tumor off the dome of the liver moving through or beneath
Glisson's capsule. Hemostasis is achieved as the dissection
proceeds using generous coagulation electrosurgery and the argon
beam electrocoagulation (Birtcher Electrosurgical, Englewood,
CO). Isolated patches of tumor on the liver surface are
electroevaporated with the distal 2 cm of the ball-tip bent and
stripped of insulation (hockey stick configuration). Ball-tip
electrosurgery is also utilized to extirpate tumor in and around
the umbilical fissure of the liver.
 |
|
Dissection beneath tumor through Glisson's capsule. From Sugarbaker
PH: Peritonectomy procedures. Ann Surg 221:29-42, 1995.
Removal of tumor from beneath the right hemidiaphragm, from
right subhepatic space, and from the surface of the liver
Tumor from beneath the right hemidiaphragm, from the right
subhepatic space, and from the surface of the liver forms an
envelope as it is removed en-bloc (Figure 16). The
dissection is greatly simplified if the tumor specimen can be
maintained intact. The dissection continues laterally on the
right to encounter the perirenal fat covering the right kidney.
Also, the right adrenal gland is visualized as tumor is stripped
out of Morrison's pouch (right sub hepatic space). Care is taken
not to traumatize the vena cava or to disrupt caudate lobe veins
that pass between the vena cava and segment 1 of the liver.
 |
|
Removal of tumor from beneath the right hemidiaphragm, from right
subhepatic space, and from the surface of the liver. From
Sugarbaker PH: Peritonectomy procedures. Ann Surg
221:29-42, 1995.
Completed right upper quadrant peritonectomy
With strong traction on the right costal margin and medial
displacement of the liver, one can visualize the completed right
upper quadrant peritonectomy. The anterior branch of the phrenic
artery and vein are seen and have been preserved (Figure 17).
The right hepatic vein and the vena cava below have been exposed.
The right adrenal gland and Gerota's fascia covering the right
kidney constitutes the base of the dissection. Not infrequently,
tumor will be densely adherent to the tendinous mid-portion of
the left or right hemidiaphragm. If this occurs, the fibrous
tissue infiltrated by tumor must be resected. This usually
requires a generous elliptical excision of a portion of the
hemidiaphragm. The defect in the diaphragm is left open until the
intraperitoneal chemotherapy is complete. Then it is closed with
interrupted sutures and rarely causes respiratory problems
postoperatively. Implantation Of tumor cells into the pleural
space must be avoided by treating the pleural space along with
the peritoneal cavity with heated intraoperative intraperitoneal
chemotherapy.
 |
|
Completed right upper quadrant peritonectomy. From Sugarbaker PH:
Peritonectomy procedures. Ann Surg 221:29-42 1995.
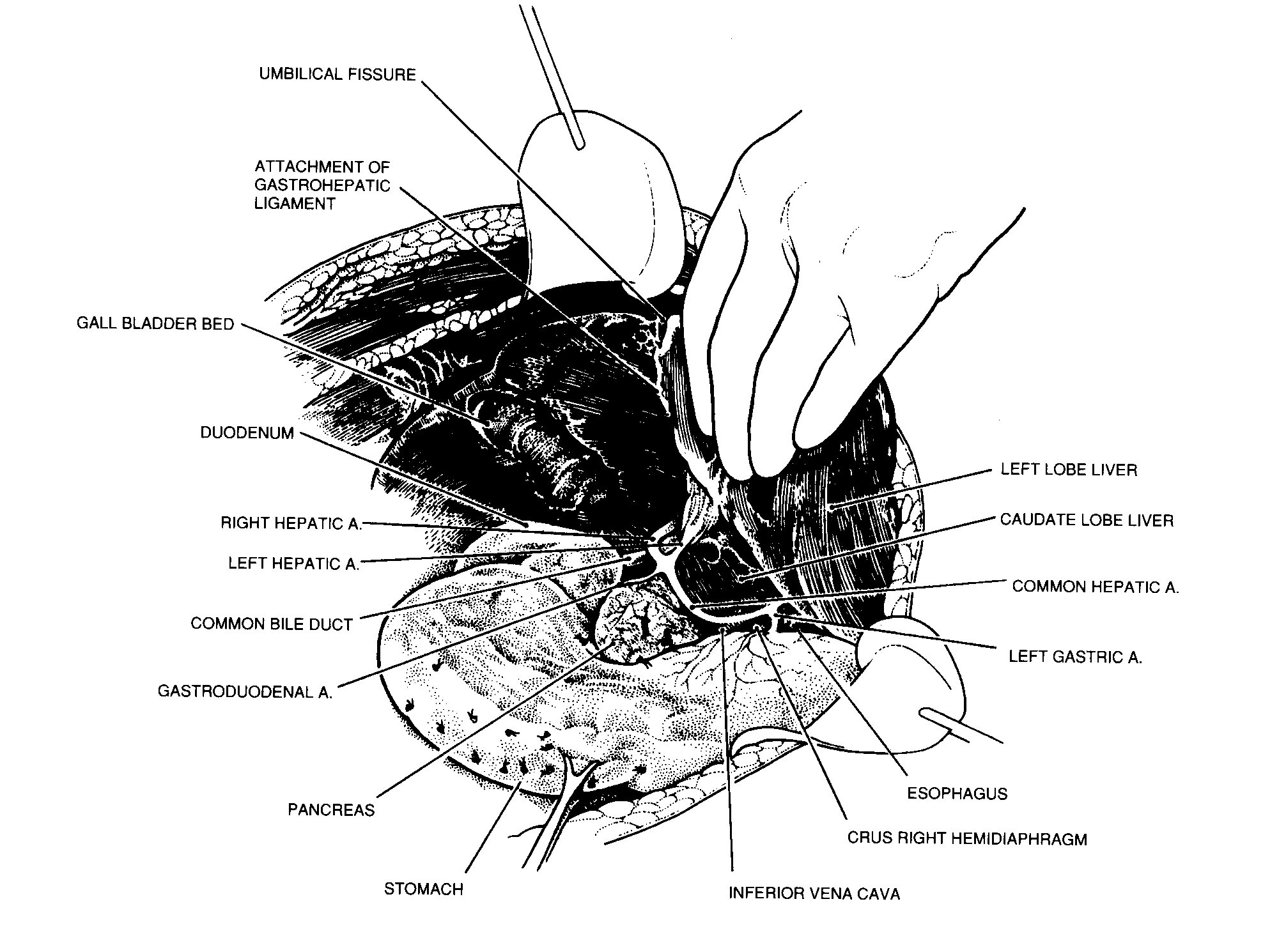
Lesser omentectomy and cholecystectomy
The gall bladder is removed in a routine fashion from its
fundus towards the cystic artery and cystic duct (Figure 18).
These structures are ligated and divided. The tissue superior to
the porta hepatis is usually heavily covered by tumor. Using
strong traction, the cancerous tissue, which covers the common
duct and hepatic artery, is fractured away from the gall bladder
bed towards the duodenum. In this dissection ball-tip
electrosurgery may be excessively traumatic; structures of the
porta hepatis are dissected free of tumor by the spreading of a
clamp. Electrocoagulation is used to divide tissues above the
clamp.
To begin resection of the lesser omentum, one dissects along the
gastrohepatic fissure that divides liver segments 2 and 3 from
segment 1. One goes back to ball-tip electrosurgery for this
maneuver and for electroevaporation of the tumor from the
anterior surface of the left caudate process. Great care is taken
not to traumatize the caudate process, for this can result in
excessive and needless blood loss.
The segmental blood supply to the caudate lobe is located on the
anterior surface of this segment of the liver, and hemorrhage may
occur with only superficial trauma. Also, one must be aware that
the left hepatic artery may arise from the left gastric artery
and cross through the hepatogastric fissure. If this occurs, one
dissects with a spreading clamp along this vessel to isolate and
preserve it.
 |
|
Lesser omentectomy and cholecystectomy. From Sugarbaker PH:
Peritonectomy procedures. Ann Surg 221.29-42, 1995.
Stripping of the omental bursa
As one clears the left part of the caudate liver segment of
tumor, the vena cava is visualized directly beneath (Figure 19).
To strip the omental bursa, strong traction is maintained on the
tumor and ball-tip electrosurgery is used to divide the fibrous
tissue above the vena cava and clear the peritoneum that lies
anterior to the crus of the right hemidiaphragm.
 |
|
Stripping of the omental bursa. From Sugarbaker PH:
Peritonectomy procedures. Ann Surg 221.29-42, 1995.
The common hepatic artery and the origin of the left gastric
artery are skeletonized and avoided. A spreading clamp with blade
electrosurgery is used to visually identify the cephalad and
caudad branching of the left gastric artery and the coronary
vein. Dissection of lesser omental fat, using pressure between
the thumb and index finger will help identify the two major
branches of the left gastric artery. They are preserved in order
to ensure adequate blood supply to the stomach. Several branches
of the left gastric artery must remain intact in order to provide
blood supply to the stomach.
The surgeon continues to dissect in a clockwise direction along
the lesser curvature of the stomach. Care is taken to preserve as
much lesser omental fat as is possible, only removing tumor
tissue. The branches of the anterior vagus nerve to the antrum of
the stomach are preserved if possible. Finally, dissection using
a spreading clamp around celiac lymph nodes allows the specimen
to be released. If the pylorus is firm and tight, a pyloroplasty
must be performed in order to allow the stomach to empty. If the
pylorus is widely open, a gastric drainage procedure will not be
needed even though a vagotomy has been performed.
Pelvic peritonectomy with resection of the rectosigmoid colon
To initiate the pelvic dissection, the peritoneum is stripped
from the posterior surface of the lower abdominal incision,
exposing the rectus muscle (Figure 20). The muscular
surface of the bladder is seen as ball-tip electrosurgery strips
tumor-bearing peritoneum and pre-peritoneal fat from this
structure. The urachus must be divided and used as a point for
traction for this dissection. Round ligaments are divided as they
enter the internal inguinal ring on both the right and left in-
the female patient.
The peritoneal incision around the pelvis is completed by
stripping peritoneum posteriorly up to the duodenum and the
Treitz ligament. Right and left ureters are identified and
preserved. In females, the right and left ovarian veins are
ligated and divided at the lower pole of the kidney. A linear
stapler is used to divide the colon at the junction of sigmoid
and descending colon. The vascular supply of the distal portion
of the bowel is traced back to its origin on the aorta. The
inferior mesenteric artery is ligated and divided. This allows
one to pack all of the viscera, including the proximal sigmoid
colon, into the upper abdomen.
 |
|
Pelvic peritonectomy. From Sugarbaker PH: Peritonectomy
procedures. Ann Surg 221:29-42, 1995.
Hysterectomy and transection of the rectum beneath the
peritoneal reflection
Ball-tip electorsurgery is used to dissect deep to the
mesorectum (Figure 21). One works in a centripetal fashion
to free up the entire pelvis. An extraperitoneal suture ligation
of the uterine arteries occurs just above the ureter and close to
the base of the bladder. In females, the bladder is gently moved
off from the cervix and the vagina is entered. The vaginal cuff
anterior and posterior to the cervix is divided using ball-tip
electrosurgery and the perirectal fat inferior to the posterior
vaginal wall is encountered. Ball-tip electrosurgery is used to
divide the perirectal fat beneath the peritoneal reflection. This
ensures that all tumor which occupies the cul-de-sac is removed
intact with the specimen. The mid-portion of the rectal
musculature is skeletonized using ball-tip electrosurgery. A
roticulator stapler (Autosuture Inc., Norwalk, CT) is used to
close the rectal stump.
Vaginal closure and colorectal anastomosis
Interrupted absorbable sutures are used to close the vaginal
cuff and prevent leakage of fluid during intraperitoneal
perfusion (Figure 22). A monofilament suture in a purse
string fashion is used to secure the stapler anvil in the
proximal sigmoid colon. A circular stapling device is passed into
the rectum and the trochar used to penetrate the middle of the
rectal staple line. The body of the circular stapler and anvil
are mated and the stapler fired to complete the low colorectal
anastomosis (Intraluminal Stapler ILS-33, Ethicon, Cincinnati,
OH). This anastomosis is performed after the heated
intraoperative intraperitoneal chemotherapy is completed.
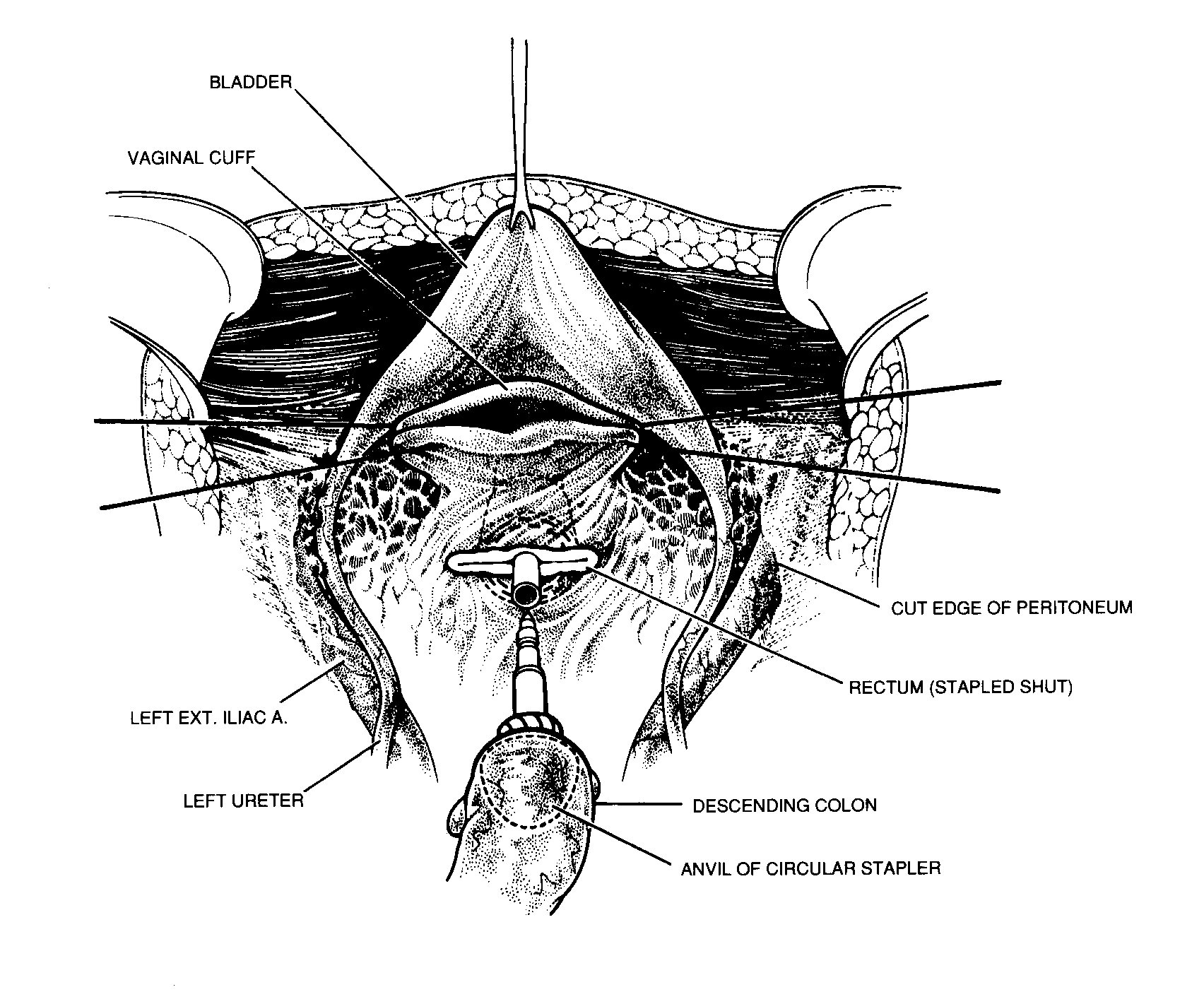 |
|
Resection of rectosigmoid colon beneath the peritoneal
reflection. From Sugarbaker PH: Peritonectomy procedures. Ann
Surg 221:29-42, 1995.
 |
|
Complete pelvic stripping and rectosigmoid resection. From
Sugarbaker PH: Peritonectomy procedures. Ann Surg
221:29-42, 1995.
An absolute requirement of a complication-free, low colorectal
anastomosis is an absence of tension on the suture line. Adequate
mobilization of the entire left colon with preservation of its
blood supply is necessary. To accomplish this requires several
steps.
The branches of the inferior mesenteric artery (superior
hemorrhoidal, sigmoid and left colic) are ligated as they arise
from this vascular trunk. This converts the Y-configuration of
these vessels to a V-configuration. This allows for great
stretching of the left colic mesentery with a preservation of
adequate blood supply to the distal colon (Figure 23).
Then the inferior mesenteric artery is ligated and then suture
ligated on the aorta. The inferior mesenteric vein is divided as
it courses around the duodenum. The mesentery of the' transverse
colon and splenic flexure are completely elevated from the
perirenal fat. Taking care to avoid the left ureter, one divides
the left colon mesentery from all of its retroperitoneal
attachments. These maneuvers will allow the junction of sigmoid
and descending colon to reach to the low rectum for a
tension-free anastomosis.
In order to ensure a safe colorectal anastomosis, one examines
the proximal and distal tissue rings for their completeness. One
insufflates air under pressure into the rectum with a
water-filled pelvis to check for staple closure and an air-tight
anastomosis: One observes that the distal colonic segment follows
the concavity of the sacrum showing that there is no tension on
the stapled anastomosis. A rectal examination is performed to
check for staple-line bleeding at the anastomosis.
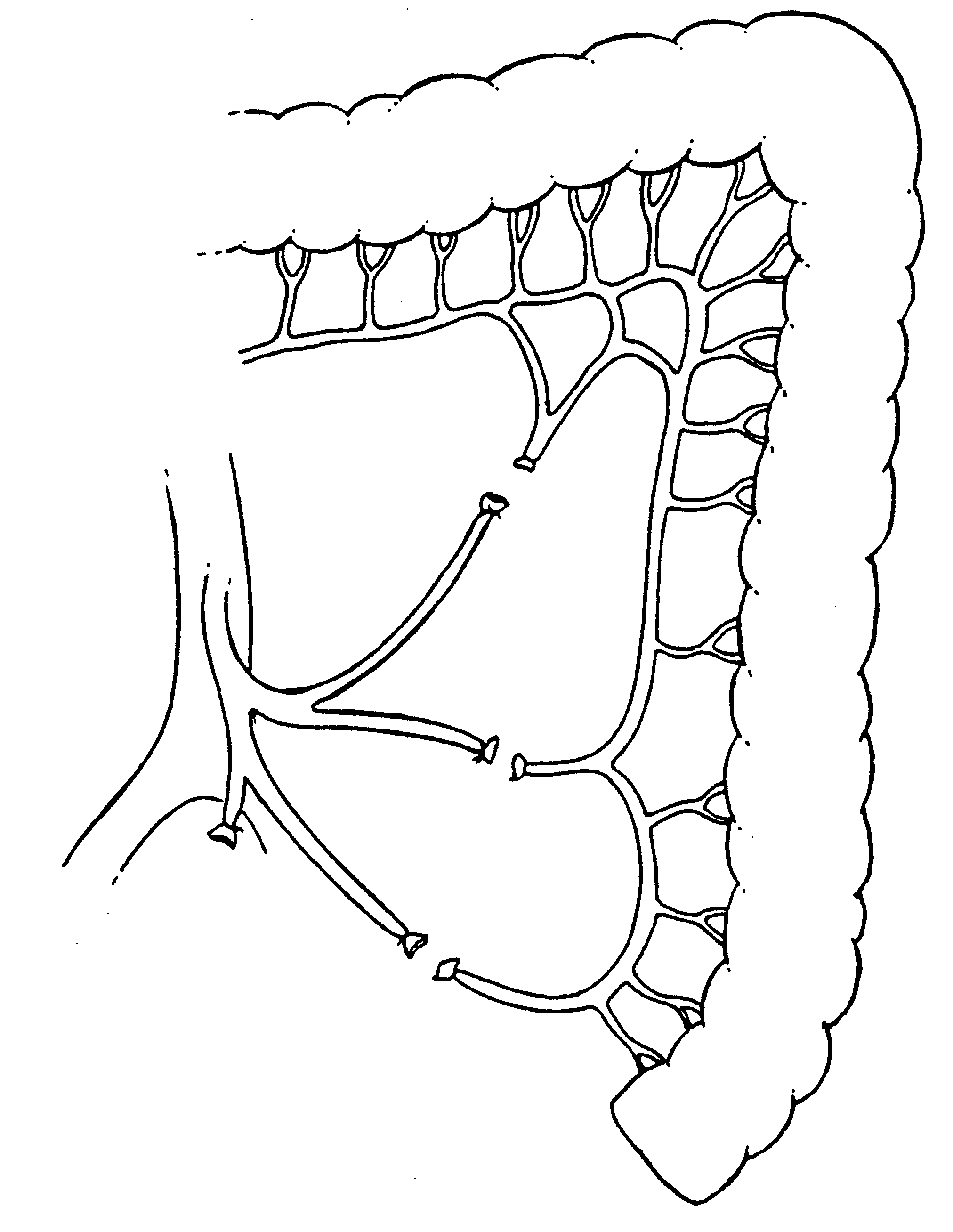 |
|
Preservation of the intermediate vasculature to the distal colon
by converting the Y-configuration of the sigmoidal vessels to a
V-configuration.
Antrectomy and gastric reconstruction
The gastric antrum, as with other motionless intraabdominal
structures, may be so densely surrounded by tumor that resection
rather than peritoneal stripping is required for complete tumor
removal. The right gastric artery is divided and blunt dissection
used to separate the first portion of the duodenum from the
pancreas. A stapler (Ethicon TLC-75. Cincinnati, OH) is used to
close off and transect the duodenum just below the last visible
evidence of tumor. Similarly, a stapler (Ethicon TA 90,
Cincinnati, OH) divides the stomach proximally.
The duodenal and gastric staple lines are inverted with
interrupted sutures and a side-to-side gastrojejunostomy is
performed (Figure 24). These suture lines and the
inversion of staple lines are not performed until the heated
intraoperative intraperitoneal chemotherapy is completed.
 |
|
Gastric reconstruction after antrectomy.
Total gastrectomy and reconstruction
In some patients, a total gastrectomy will be needed to clear
the left upper quadrant of mucinous tumor. In most instances,
this indicates that the tumor is a more aggressive type, usually
called pseudomyxoma/carcinoma hybrid. Alternatively, the patient
may have had many prior surgical procedures with prior extensive
dissection in the left upper quadrant.
To perform the gastrectomy, the esophagus is closed with a linear
stapler (Ethicon TA-30, Cincinnati, OH) and then transected. The
duodenum is transected just distal to the pylorus with a linear
stapler. The left gastric artery and vein are ligated and suture
ligated. Final attachments of the stomach to celiac lymph nodes
and to the superior portion of the head of the pancreas are
divided using ball-tipped electrosurgery. Great care is taken not
to traumatize the anterior surface of the pancreas.
To reconstruct the gastrointestinal tract after gastrectomy that
is part of a complete cytoreduction, a duodenal exclusion
operation is performed. This protects the esophagojejunal
anastomosis. Approximately 20 cm below the ligament of Treitz a
portion of jejunum is transected with a linear stapler and
brought in a retrocolic fashion up to the esophagus. The
esophageal staple line is removed and a purse-string suture is
used to secure the anvil of a circular stapler in the distal
esophagus (Ethicon ILS-29, Cincinnati, OH). The staple line
closing the proximal jejunum is removed and the stapler is passed
approximately 5 cm into the jejunum and then the spear is passed
through the jejunal wall. It is mated with the anvil within the
esophagus, and the staple line is completed. The proximal jejunum
is stapled off, and then the staple line inverted with
interrupted sutures. All of these suture lines are performed
after the heated intraoperative intraperitoneal chemotherapy has
been completed.
The portion of jejunum proximal to the linear staple line is now
brought out in the left upper quadrant as an end ostomy in order
to divert all bile and digestive enzymes from the
gastrointestinal tract. This diverting jejunostomy is closed
between 3 and 6 months postoperatively as part of a second-look
procedure (Figure 25).
 |
|
In patients who require gastrectomy in addition to extensive
cytoreduction, a high diverting jejunostomy is performed. This
ostomy is closed in approximately six months with a second-look
surgery.
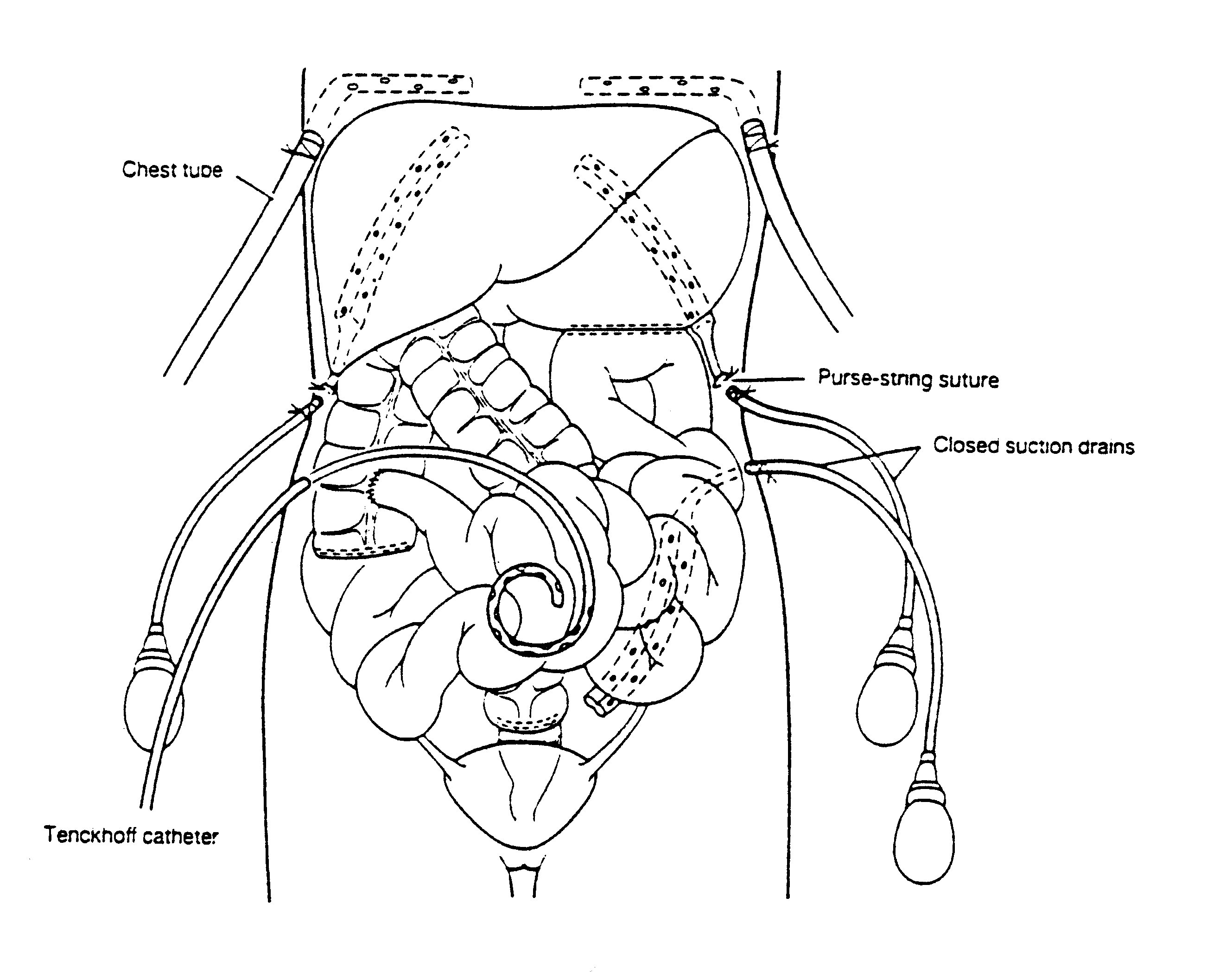
Tubes and drains required for heated intraoperative and early
postoperative intraperitoneal chemotherapy
Closed suction drains are placed in the dependant portion of
the abdomen. This includes the right subhepatic space, the left
subdiaphragmatic space and the pelvis.
 |
|
Tubes and drains required for heated intraoperative and early
postoperative intraperitoneal chemotherapy. From Sugarbaker PH:
Peritonectomy procedures. Ann Surg 221:29-42, 1995.
A Tenckhoff catheter (Quinton Curled Peritoneal Catheter,
Quinton, Inc., Seattle, WA) is placed through the abdominal wall
in order to administer heated intraoperative intraperitoneal
chemotherapy (Figure 26). All transabdominal drains and
tubes are secured in a watertight fashion with a purse string
suture at the skin. Right angle thoracostomy tubes (Deknatel,
Floral Park, NY) are inserted on both the right and left side in
order to prevent fluid accumulation in the chest as a result of
intraperitoneal chemotherapy and diaphragm stripping.
As soon as the abdomen is closed, irrigation of the abdomen with
1.5% dextrose peritoneal dialysis solution (Abbott Laboratories,
Chicago, IL) is begun. Standardized orders for early
postoperative intraperitoneal lavage and for perioperative
intraperitoneal chemotherapy administration are instituted (Tables
6 and 7).
Title Page | Introduction | Principles
of Intraperitoneal Chemotherapy | Current Indications for
Cytoreductive Surgery and Intraperitoneal Chemotherapy
Heated
Intraoperative Intraperitoneal Chemotherapy by the Coliseum
Technique
Immediate
Postoperative Abdominal Lavage in Preparation for Early
Postoperative Intraperitoneal 5-Fluorouracil
Early
Postoperative Intraperitoneal Chemotherapy for Adenocarcinoma | Induction
Intraperitoneal Chemotherapy for Debilitating Ascites
Cytoreductive
Surgery for Peritoneal Surgacy Malignancy - Pertitonectomy
Procedures | Results of Treatment of
Peritoneal Surface Malignancy
Conclusions | References