|
 |
|
Title Page | Introduction | Principles
of Intraperitoneal Chemotherapy | Current Indications for
Cytoreductive Surgery and Intraperitoneal Chemotherapy
Heated
Intraoperative Intraperitoneal Chemotherapy by the Coliseum
Technique
Immediate
Postoperative Abdominal Lavage in Preparation for Early
Postoperative Intraperitoneal 5-Fluorouracil
Early
Postoperative Intraperitoneal Chemotherapy for Adenocarcinoma | Induction
Intraperitoneal Chemotherapy for Debilitating Ascites
Cytoreductive
Surgery for Peritoneal Surgacy Malignancy - Pertitonectomy
Procedures | Results of Treatment of
Peritoneal Surface Malignancy
Conclusions | References
I. PRINCIPLES OF INTRAPERITONEAL CHEMOTHERAPY
Intraperitoneal chemotherapy gives high response rates within
the abdomen because the peritoneal space to plasma barrier
provides dose intensive therapy (1). Figure 1 shows that
large molecular weight substances, such as mitomycin C. are
confined to the abdominal cavity for long time periods (2). This
means that the exposure of peritoneal surfaces to
pharmacologically active molecules can be increased considerably
by giving the drugs via the intraperitoneal as opposed to
intravenous route.
|
 |
|
Large molecular weight compounds when instilled into the
peritoneal cavity are sequestered at that site for long periods.
The physiologic barrier to the release of intraperitoneal drugs
is called the peritoneal space to plasma barrier. In this
experiment, 15 mg of mitomycin C was infused into the peritoneal
cavity as rapidly as possible. Intraperitoneal, intravenous,
portal venous and urine mitomycin C concentrations were
determined by HPLC assay. (From Sugarbaker PH, Graves T, DeBruijn
EA, Cunliffe WJ, Mullins RE, Hull WE, Oliff L, Schlag P:
Rationale for Early Postoperative Intraperitoneal Chemotherapy
(EPIC) in patients with advanced gastrointestinal cancer. Cancer
Research 50:5790-5794, 1990.
For the chemotherapy agents used to treat peritoneal
carcinomatosis or peritoneal sarcomatosis, the area under the
curve (AUC) ratios of intraperitoneal to intravenous exposure are
favorable. Table 1 presents the AUC IP/IV for the drugs in
routine clinical use in patients with peritoneal seeding (2,3).
In our studies, these include 5-fluorouracil, mitomycin C,
doxorubicin, and cisplatin.
TABLE 1 |
||
Area under
the curve ratios of peritoneal surface exposure to
systemic exposure for drugs |
||
| Drug | Molecular Weight |
Area Under the Curve Ratio |
| 5-Fluorouracil Mitomycin C Doxorubicin Cisplatin |
130 334 544 300 |
250 75 500 20 |
Sugarbaker and colleagues have advanced the tumor cell
entrapment hypothesis to explain the high incidence of peritoneal
seeding in patients who undergo the surgical treatment of
intraabdominal adenocarcinoma or sarcoma. This theory relates the
high incidence of tumor implantation to one or more of the
following mechanisms:
| a. free intraperitoneal tumor emboli as a result of
full thickness invasion of the bowel wall by cancer; b. leakage of malignant cells from transected lymphatic channels; c. dissemination of malignant cells from trauma as a result of surgical dissection; d. blood clots that remain in the abdomen or pelvis that contain viable cancer cells; e. fibrin entrapment of intraabdominal tumor emboli on traumatized peritoneal surfaces; f. tumor promotion of these entrapped cells through growth factors involved in the wound healing process. |
In order to interrupt implantation of tumor cells on
intraabdominal and pelvic surfaces, the abdominal cavity may be
flooded with chemotherapy in a large volume of fluid prior to
surgery (induction chemotherapy). during surgery (heated
intraoperative intraperitoneal chemotherapy) and in the
postoperative period (early postoperative intraperitoneal
chemotherapy). Some patients with a poor prognosis may be
recommended for adjuvant systemic chemotherapy. These strategies
may be used to treat peritoneal surface malignancy or to prevent
it in groups at high risk. In this approach to peritoneal surface
malignancy, there is a change in the route (intraperitoneal vs.
intravenous) and timing (perioperative vs. postoperative) of
chemotherapy delivery.
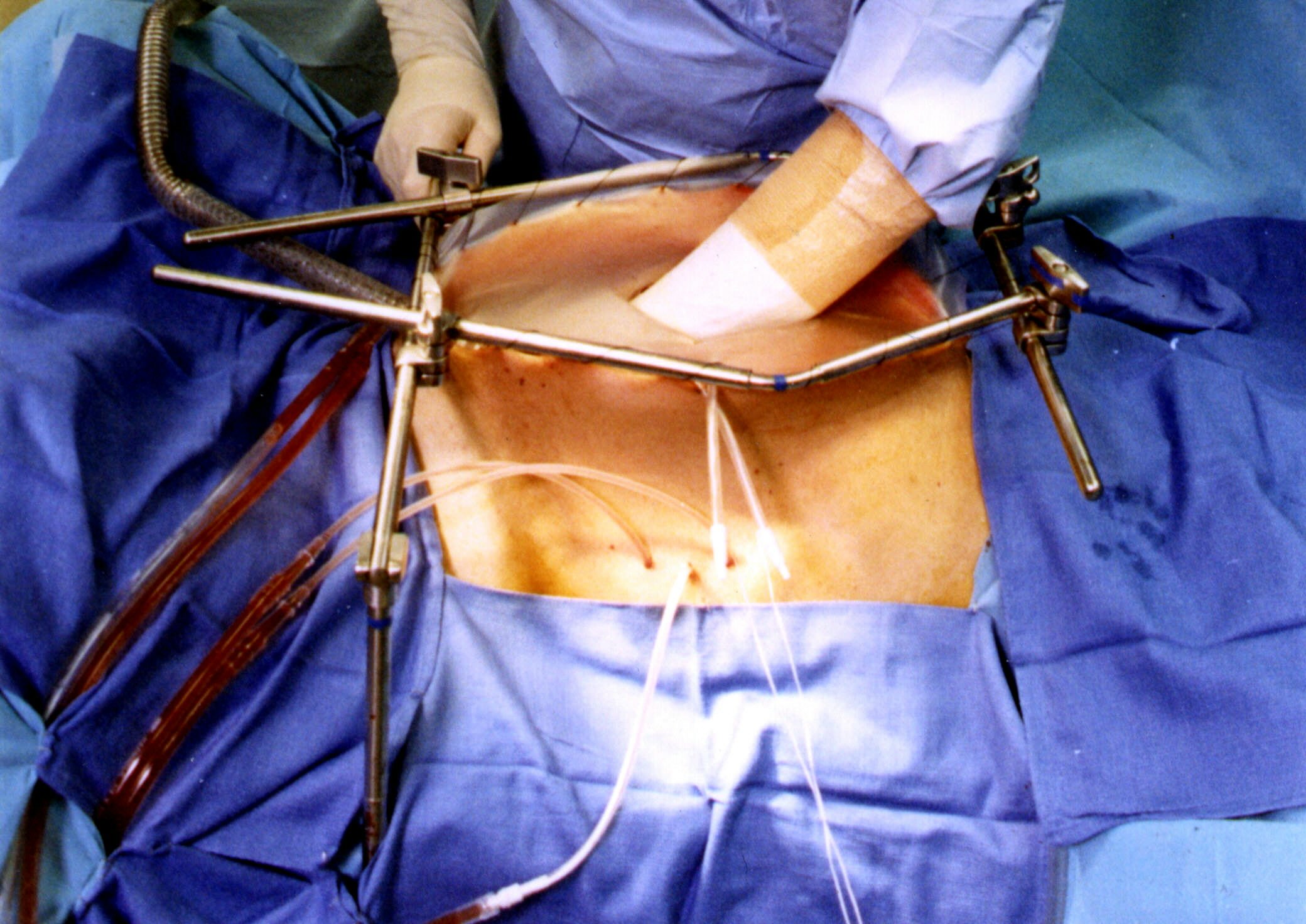
This new approach to the surgical treatment of intraabdominal
malignancy begins in the operating room after a complete
resection of a primary cancer or after the cytoreduction of a
cancer with carcinomatosis or sarcomatosis. The intraoperative
chemotherapy is performed prior to construction of suture lines.
A proper placement of tubes, drains and temperature probes is
needed prior to initiation of intraperitoneal chemotherapy.
Before abdominal closure, the temperature probes are removed, but
the tubes and drains may be required for early postoperative
intraperitoneal lavage and chemotherapy.
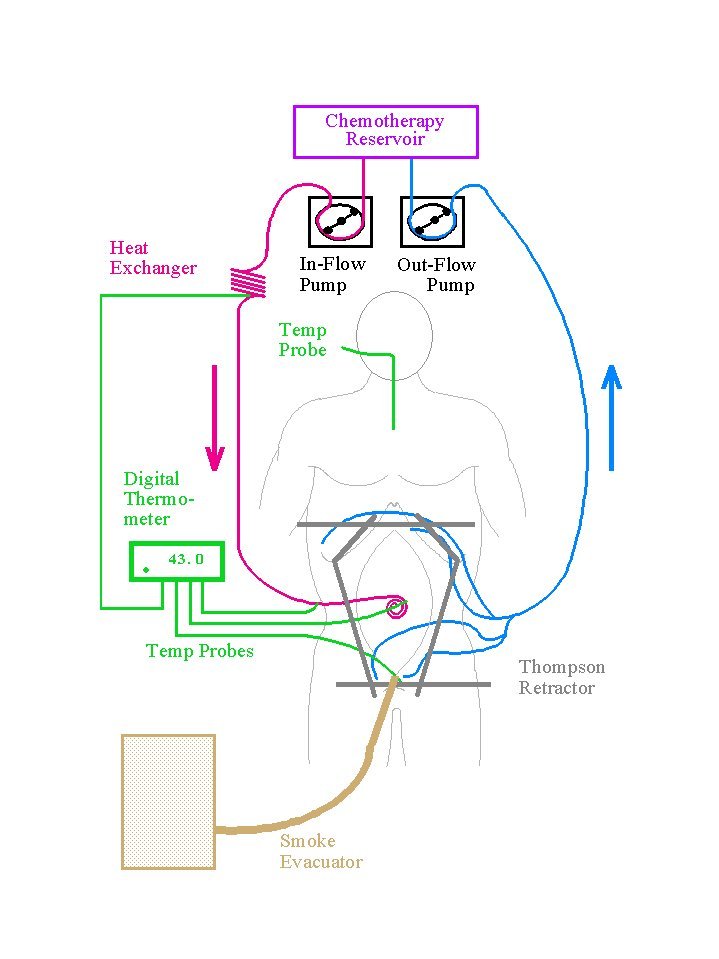
In the operating room, heated intraperitoneal chemotherapy is
used. Heat is part of the optimizing process and is used to bring
as much dose intensity to the abdominal and pelvic surfaces as is
possible. Hyperthermia with intraperitoneal chemotherapy has
several advantages. First, heat by itself has greater toxicity
for cancerous tissue than for normal tissue. This predominant
effect on cancer increases as the vascularity of the malignancy
decreases. Second, hyperthermia increases the penetration of
chemotherapy into tissues. As tissues soften in response to heat,
the elevated interstitial pressure of a tumor mass may decrease
allowing improved drug penetration. Third, and probably most
important, heat increases the cytotoxicity of selected
chemotherapy agents. This synergism occurs only at the interface
of heat and body tissue, at the peritoneal surface. The benefits
of heat and the intraoperative timing of intraperitoneal
chemotherapy are listed in Table 2.
In the immediate postoperative period, an abdominal lavage
removes tissue debris and blood products from the abdominal
cavity to minimize fibrin accumulation. Also, the lavage
maintains the patency of the closed suction drains.
Tumor cells that remain in the abdominal cavity can be destroyed
by the pharmacologic concentrations of intraperitoneal
chemotherapy instilled on postoperative days 1 through 5. The
timely use of intraperitoneal chemotherapy in the early
postoperative period eliminates tumor cells from the abdomen
before they are fixed within scar tissue that results from wound
healing.
The chemotherapy not only directly destroys tumor cells but it
also eliminates viable platelets, neutrophils and monocytes from
the peritoneal cavity. This diminishes the promotion of tumor
growth associated with the wound healing process. However,
removal of the white blood cells also decreases the ability of
the abdomen to resist infection. For this reason, strict aseptic
technique is imperative when administering the chemotherapy or
handling abdominal tubes and drains.
TABLE 2 |
Benefits of intraoperative timing of intraperitoneal chemotherapy |
|
Prognostic groups of patients with peritoneal carcinomatosis
The prognostic features that control the results of treatment
in patients with peritoneal carcinomatosis have been determined.
Prognostic indicators include: (1) the grade of the malignant
tumor, (2) the presence or absence of lymphatic or hematogenous
metastases, and (3) the completeness of the surgical removal of
cancer from the abdomen and pelvis. Table 3 presents the
prognostic groups for peritoneal carcinomatosis from colon
cancer, rectal cancer, and appendiceal cancer now being used
clinically to predict outcome. For invasive malignancy, a
complete cytoreduction indicates that no visible nodules of
cancer remain after surgery. For noninvasive malignancy such as
pseudomyxoma peritonei, complete cytoreduction may include
residual tumor nodules up to 2.5 mm in diameter.
TABLE 3 |
||||
Prognostic groups for peritoneal carcinomatosis |
||||
Prognostic |
Mucinous |
|
Completeness of Cytoreduction |
Expected |
I |
I |
None |
Complete |
90% |
II |
II or III |
None |
Complete |
60% |
III |
Any |
Present |
Complete |
30% |
IV |
Any |
Any |
Incomplete |
10% |
Predicting outcome for mucinous adenocarcinoma by preoperative
CT of the abdomen and pelvis
CT is an inaccurate test by which to quantitate peritoneal
carcinomatosis from adenocarcinoma (4). The malignant tissue
progresses on the peritoneal surfaces and its shape conforms to
the normal contours of the abdominal and pelvic structures. This
is quite different from the metastatic processes in liver or
lung, which progress as 3-dimensional tumor nodules.
The CT has been of greater help in locating and quantifying
mucinous adenocarcinoma within the peritoneal cavity (5). These
tumors produce a copious colloid material that is readily
distinguished by shape and by density from normal structures.
Using two distinctive radiologic criteria, those patients with
resectable mucinous peritoneal carcinomatosis can be selected
from those with non-resectable malignancy. This spares patients
who are unlikely to benefit from reoperative surgery from
unnecessary procedures.
The two radiologic criteria found to be most useful are: (1)
Segmental obstruction of small bowel, and (2) Presence of tumor
more than 5 cm in greatest dimension on the small bowel surface
or directly adjacent to small bowel mesentery. These criteria
reflect radiologically the biology of the mucinous
adenocarcinoma. The obstructed segments of small bowel signal an
invasive character of malignancy that is unlikely to be
cytoreduced completely. The mucinous cancer on small bowel and
small bowel mesentery indicates that the mucinous cancer is no
longer redistributed. This means that small bowel surfaces will
have residual disease after cytoreduction, for this surface is
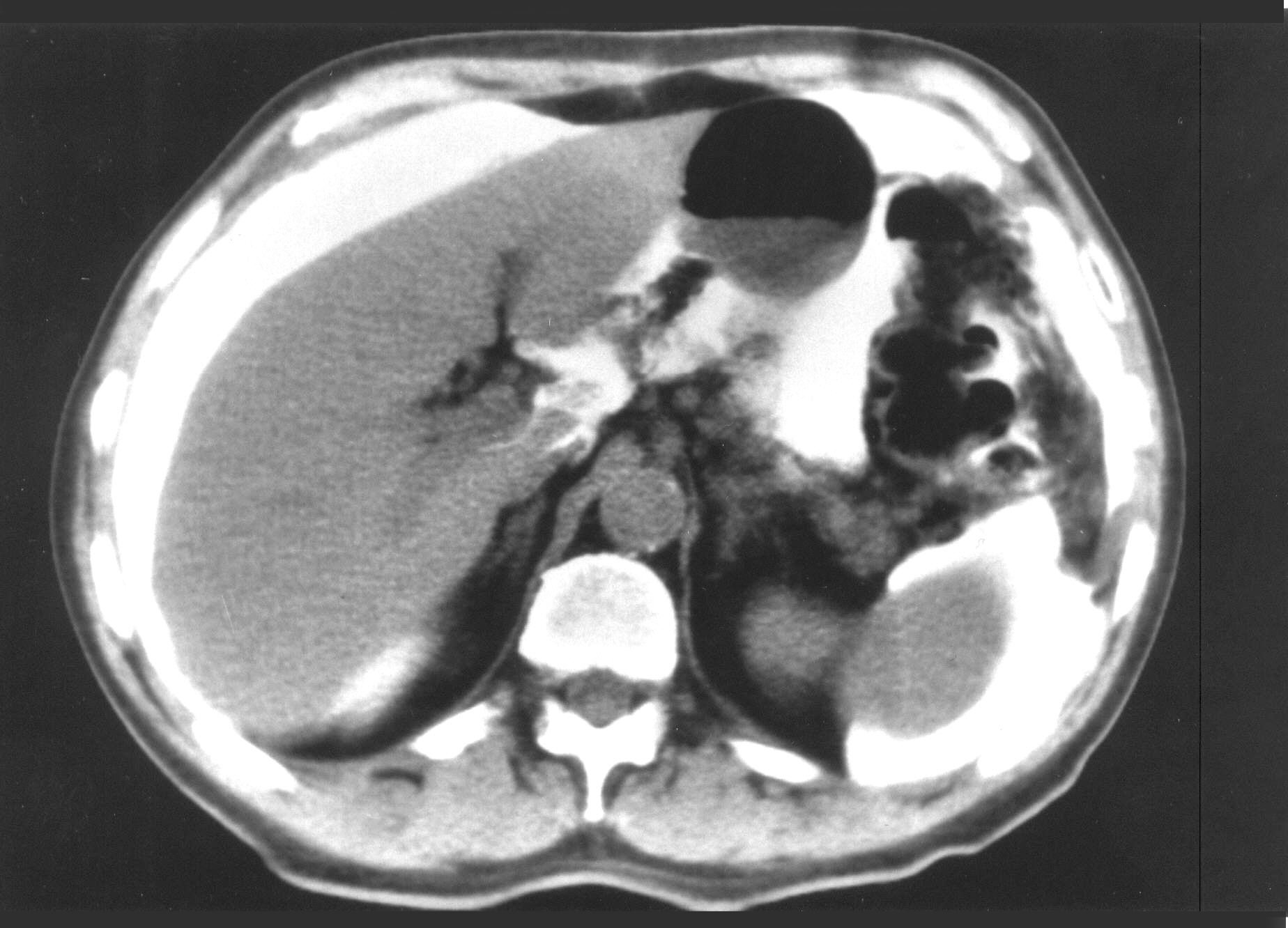
difficult to peritonectomize (see Figures 2 and 3).
 |
|
A patient with noninvasive mucinous adenocarcinoma of appendiceal
origin (pseudomyxoma peritonei) who had a complete cytoreduction
and remains disease-free at four years postoperatively. The
mucinous tumor is very extensive but the small bowel loops are of
normal caliber and are not distended by air. Also, the small
bowel has become "compartmentalized" by the mucinous
tumor. The small bowel surfaces and small bowel mesentery remain
free of tumor.
 |
|
A patient with aggressive mucinous adenocarcinoma who recurred
after extensive prior cytoreductive surgery. Small bowel loops
are slightly distended, contain small volumes of air, and its
mesenteric surface is coated by mucinous tumor nodules. This
patient has less than 5% likelihood of a complete cytoreduction.
Title Page | Introduction | Principles
of Intraperitoneal Chemotherapy | Current Indications for
Cytoreductive Surgery and Intraperitoneal Chemotherapy
Heated
Intraoperative Intraperitoneal Chemotherapy by the Coliseum
Technique
Immediate
Postoperative Abdominal Lavage in Preparation for Early
Postoperative Intraperitoneal 5-Fluorouracil
Early
Postoperative Intraperitoneal Chemotherapy for Adenocarcinoma | Induction
Intraperitoneal Chemotherapy for Debilitating Ascites
Cytoreductive
Surgery for Peritoneal Surgacy Malignancy - Pertitonectomy
Procedures | Results of Treatment of
Peritoneal Surface Malignancy
Conclusions | References