 |
Figure 22 |
I. Introduction II.Principles
of Management III. Current
Methodologies for Delivery of Intraperitoneal Chemotherapy
IV. Clinical
Results of Treatment V. Ethical Considerations
in Clinical Studies with Peritoneal Surface Malignancy VI.
References
III. Current Methodology for Delivery of Intraperitoneal Chemotherapy
Hyperthermic Intraoperative Intraperitoneal Chemotherapy Administration
In the operating room, heated intraoperative intraperitoneal chemotherapy is used. Heat is part of the optimizing process and is used to bring as much dose intensity to the abdominal and pelvic surfaces as is possible. Hyperthermia with intraperitoneal chemotherapy has several advantages. First, heat by itself has more toxicity for cancerous tissue than for normal tissue. This predominant effect on cancer increases as the vascularity of the malignancy decreases. Second, hyperthermia increases the penetration of chemotherapy into tissues. As tissues soften in response to heat, the elevated interstitial pressure of a tumor mass may decrease and allow improved drug penetration. Third, and probably most important, heat increases the cytotoxicity of selected chemotherapy agents. This synergism occurs only at the interface of heat and body tissue at the peritoneal surface. The rationale for using heated chemotherapy as a surgically directed modality in the operating room is presented in Table 2.
Rationale for the Use of Hyperthermic Intraoperative Intraperitoneal Chemotherapy |
||
|
||
Table 2 |
||
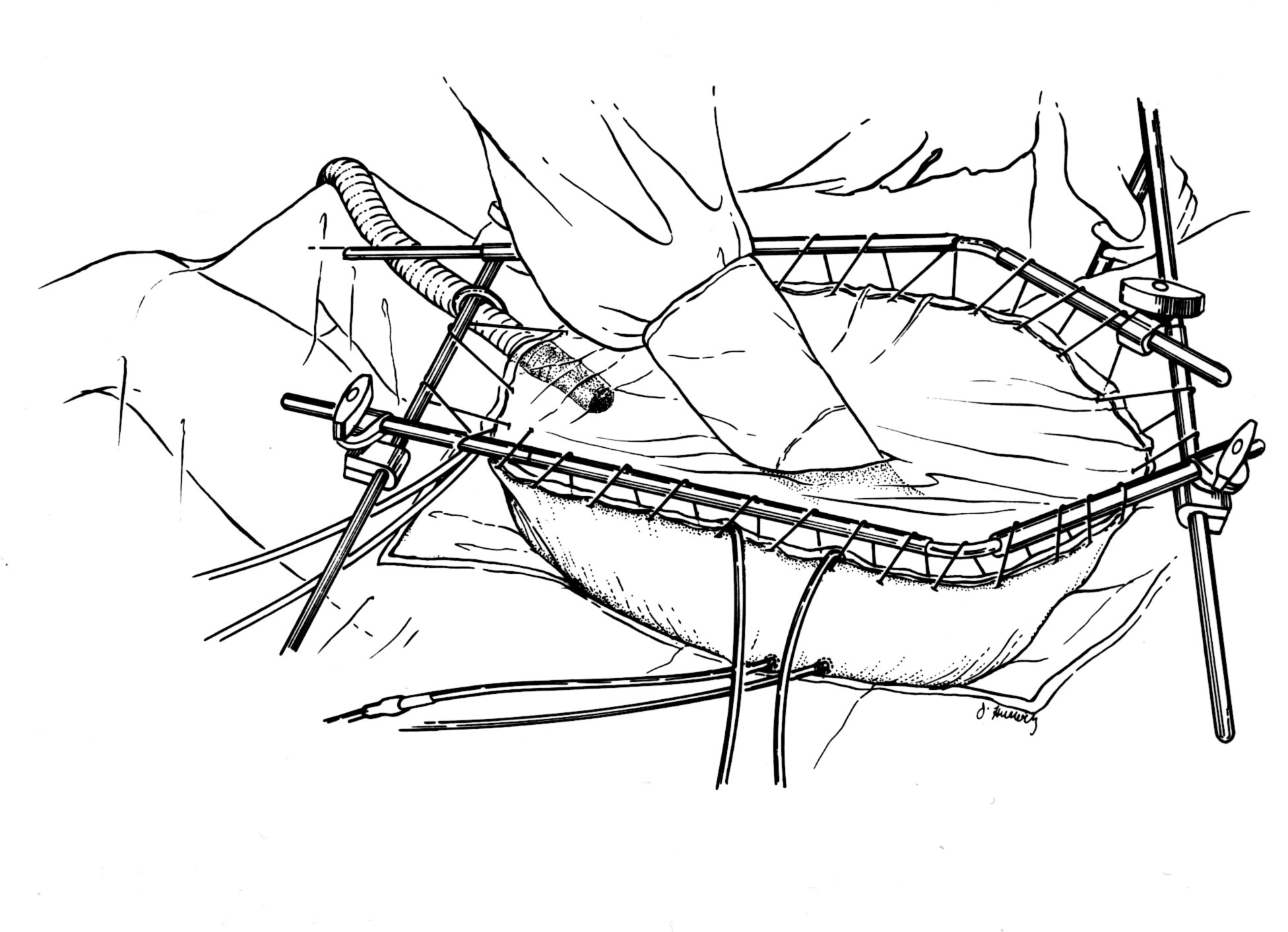
After the cancer resection is complete, the Tenckhoff catheter and closed suction drains are placed through the abdominal wall and made watertight with a purse string suture at the skin. Temperature probes are secured to the skin edge. Using a long running #2 monofilament suture, the skin edges are secured to the self-retaining retractor. A plastic sheet is incorporated into these sutures to create a covering for the abdominal cavity. A slit in the plastic cover is made to allow the surgeon’s double-gloved hand access to the abdomen and pelvis. (Figure 22) During the 90 minutes of perfusion, all the anatomic structures within the peritoneal cavity are uniformly exposed to heat and to chemotherapy. The surgeon gently but continuously manipulates all viscera to keep adherence of peritoneal surfaces to a minimum. If bleeding or some other event occurs, the chemotherapy solution is suctioned into the reservoir so that full visualization of the abdomen and pelvis is achieved. Roller pumps force the chemotherapy solution into the abdomen through the Tenckhoff catheter and pulls it out through the drains. A heat exchanger keeps the fluid being infused at 44oC-46oC so that the intraperitoneal fluid is maintained at 42oC-43oC. The apparatus used for the administration of heated intraoperative intraperitoneal chemotherapy is diagrammed in Figure 23. The smoke evacuator is used to pull air from beneath the plastic cover through activated charcoal, preventing contamination of air in the operating room by chemotherapy aerosols.
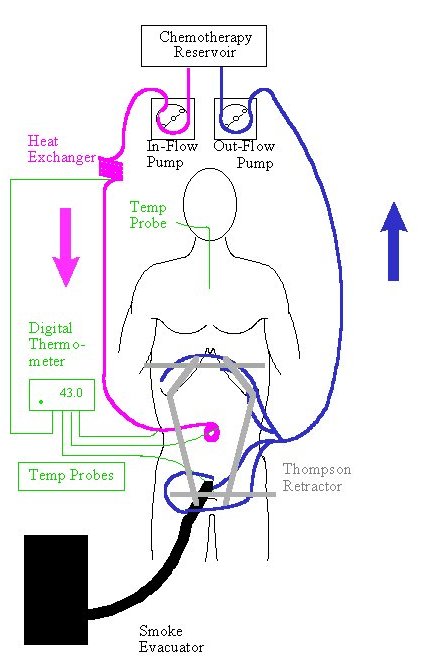 |
Figure 23 |
After the intraoperative perfusion is complete, the abdomen is suctioned dry of fluid. The abdomen is then reopened, retractors repositioned, and reconstructive surgery is performed. It should be re-emphasized that no suture lines are constructed until after the chemotherapy perfusion is complete. One exception to this rule is closure of the vaginal cuff to prevent intraperitoneal chemotherapy leakage. The standardized orders for heated intraoperative intraperitoneal chemotherapy are given in Table 3.
Standardized Orders for Hyperthermic Intraoperative Intraperitoneal Chemotherapy |
Mitomycin C
Orders
Cisplatin and Doxorubicin Orders
|
Table 3 |
Mitomycin C
Mitomycin C is used intraoperatively to treat appendiceal, colonic, and gastric cancer.
Cisplatin and Doxorubicin
Doxorubicin and cisplatin together are used to treat sarcomatosis and peritoneal mesothelioma. Also, papillary serous cancer and primary peritoneal adenocarcinoma are treated with the doxorubicin and cisplatin regimen.
Immediate Postoperative Abdominal Lavage
In patients who are to receive early postoperative intraperitoneal 5-fluorouracil, the catheters for drug instillation and abdominal drainage must be kept clear of blood clots and tissue debris. To accomplish this, an abdominal lavage is begun in the operating room. This lavage utilizes the tubes and for heated intraoperative intraperitoneal chemotherapy. Large volumes of fluid are rapidly infused and then drained from the abdomen after a short dwell time. The standardized orders for immediate postoperative abdominal lavage are given in Table 4. All intraabdominal catheters are withdrawn before the patient is discharged from the hospital.
Immediate Postoperative Abdominal Lavage |
Day of
Operation:
|
Table 4 |
Early Postoperative Intraperitoneal 5-Fluorouracil
The standardized orders for early postoperative intraperitoneal 5-fluorouracil are presented in Table 5. After the patient stabilizes postoperatively, and after the drainage from the immediate postoperative abdominal lavage is no longer blood stained, the 5-fluorouracil instillation occurs. The patients treated are those with adenocarcinoma from appendiceal, colonic and gastric primaries. Patients with pseudomyxoma peritoneal are not treated with 5-fluorouracil because the intraoperative heated mitomycin is usually sufficient. In some patients who have extensive small bowel trauma from lysis of adhesions, the early postoperative 5-fluorouracil is withheld for fear of fistula formation. ( 22 ,23 ) These patients are usually recommended for a second-look surgery.
Early Postoperative Intraperitoneal Chemotherapy with 5-Fluorouracil |
Postoperative
Days 1-5
|
Table 5 |
Induction Treatment with Intravenous Mitomycin C and Intraperitoneal 5-fluorouracil
Patients should be carefully selected to receive induction intraperitoneal chemotherapy. Intestinal adhesions are the most frequent contraindication to its use. Usually, induction intraperitoneal chemotherapy is not recommended in patients with extensive prior surgery. The treatment is directed at the small bowel surfaces and is designed to eradicate large numbers of minute peritoneal implants. If this goal can be accomplished with the induction intraperitoneal chemotherapy, then the cytoreduction will be greatly facilitated. Parietal peritoneal surfaces, stomach surfaces and the large bowel can usually be completely cytoreduced. Small bowel surfaces are the most common site for residual disease that prevents the CC-0 or CC-1 cytoreduction. Table 6 presents the standardized orders for induction chemotherapy for adenocarcinoma.
Induction Intraperitoneal 5-Fluorouracil and Intravenous Mitomycin C Chemotherapy |
|
Table 6 |
Reoperative Surgery Plus Additional Intraperitoneal Chemotherapy.
As the clinical data regarding treatment of peritoneal surface malignancy becomes available, the need for additional operative procedures and additional cycles of intraperitoneal chemotherapy become clear. This seems most evident, at this point in time, with the tumors that do not have a tumor marker by which to monitor for recurrent disease. Peritoneal carcinomatosis from colon cancer is now routinely managed with a second-look surgery at six to nine months. Also, primary peritoneal surface malignancy, especially mesothelioma, has a scheduled second-look surgery at six to nine months.
At the second-look surgery, the abdomen is widely opened and all of the peritoneal surfaces are visualized with a complete take-down of all adhesions. Additional cytoreduction is performed, and additional visceral resections may be required. If a CC-1 cytoreduction can again be achieved, then heated intraoperative intraperitoneal chemotherapy is used. If the patient has adenocarcinoma, then early postoperative intraperitoneal 5-fluorouracil is recommended.
If it appears from the re-operation that the initial heated chemotherapy and early postoperative chemotherapy treatments were successful for the most part, then the same regimen will be employed again. If there is a "chemotherapy failure" and recurrent disease is seen in areas that have been previously peritonectomized, then a chemotherapy change would be initiated.
Oncologic Emergency
As a primary gastrointestinal cancer is resected, intraperitoneal dissemination of cancer cells may sometimes occur. Of course, if a perfusionist is available to administer heated intraoperative intraperitoneal, then this would be the preferred treatment. However, in many instances a perfusion system may not be available, or it may in an emergency situation thought to be unsafe. Resections of gastrointestinal cancers may occur in which there is "intraoperative tumor spill". In these patients treatments to eliminate small volumes of microscopic residual disease are advisable. Other clinical situations that would call for the emergency use of intraoperative intraperitoneal chemotherapy would be the presence of a small volume of localized cancer seeding that would be resected as part of the removal of the primary tumor. Another indication would be a perforated intraabdominal malignancy when that perforation is through the cancer itself. Positive peritoneal cytology would also be considered an indication for the oncologic emergency.
In this treatment, the abdominal wall is elevated on a self-retaining retractor and a plastic sheet incorporated into the skin sutures. Drains which are placed for fluid removal postoperatively are used to introduce the three liters of normothermic chemotherapy. Blood warmers are generally used to bring the fluid up to body temperature. For one hour, the surgeon manipulates all of the peritoneal surfaces using generous abrasion of the peritoneal surfaces that are at greatest risk for the adherence of cancer cells. The doses of drug are the same as for heated intraoperative intraperitoneal chemotherapy. (Table 3) After the peritoneal surfaces are treated, then suture lines are constructed and the abdomen is closed. A majority of these patients will be recommended for a second-look surgery at six to nine months.
I. Introduction II.Principles
of Management III. Current
Methodologies for Delivery of Intraperitoneal Chemotherapy
IV. Clinical
Results of Treatment V. Ethical Considerations
in Clinical Studies with Peritoneal Surface Malignancy VI.
References