 |
Figure 1 |
I. Introduction II.Principles of Management III. Current
Methodologies for Delivery of Intraperitoneal Chemotherapy
IV. Clinical
Results of Treatment V. Ethical Considerations
in Clinical Studies with Peritoneal Surface Malignancy VI.
References
II. Principles of Management
The successful treatment of peritoneal surface malignancy requires a combined approach that utilizes peritonectomy procedures and perioperative intraperitoneal chemotherapy. In addition, knowledgeable patient selection is mandatory. The visceral and parietal peritonectomy procedures that one must utilize in an attempt to resect all visible evidence of disease are illustrated below. Complete cytoreduction is essential for treatment of perioneal surface malignancy to result in long-term survival. One-to-six peritonectomy procedures may be required.(6) Their utilization depends on the distribution and extent of invasion of the malignancy disseminated within the peritoneal space.
A. Peritonectomy Procedures
If a surgeon elects to manage patients with peritoneal surface malignancy, additional knowledge concerning cancer dissemination on peritoneal surfaces and numerous refinements of his technical skills are required. He must be proficient in dissection using lasermode electrosurgery.
Rationale for Peritonectomy Procedures
Peritonectomy procedures are necessary if one is to successfully treat peritoneal surface malignancies with curative intent. Peritonectomy procedures are used in the areas of visible cancer progression in an attempt to leave the patient with only microscopic residual disease. Small tumor nodules are removed using electroevaporation. Involvement of visceral peritoneum frequently requires resection of a portion of the stomach, small intestine, or colorectum.
Locations of Peritoneal Surface Malignancy
Peritoneal surface malignancy tends to involve the visceral peritoneum in greatest volume at three definite sites. These are sites where the bowel is anchored to the retroperitoneum and peristalsis results in less motion of the visceral peritoneal surface. The rectosigmoid colon, as it comes up out of the pelvis, is a non-mobile portion of the bowel. Also, it is a dependent site; and therefore, it frequently requires resection. Usually a complete pelvic peritonectomy involves stripping of the abdominal sidewalls, the peritoneum overlying the bladder, the cul-de-sac, and the rectosigmoid colon. The ileocecal valve is another area where there is limited mobility. Resection of the terminal ileum and a small portion of the right colon is often necessary. A final site often requiring resection is the antrum of the stomach. The antrum of the stomach is fixed to the rectoperitoneum at the pylorus. Tumor coming into the Foramen of Winslow accumulates in the subpyloric space and may cause intestinal obstruction as a result of gastric outlet obstruction. Occasionally, tumor in the lesser omentum will cause a confluence of disease on the lesser curvature. This may require total gastrectomy because of encasement of the vascular supply to the stomach.
Lasermode Electrosurgery
In order to adequately perform cytoreductive surgery, the surgeon must use lasermode electrosurgery. Peritonectomies and visceral resections using the traditional scissor and knife dissection will unnecessarily disseminate a large number of tumor emboli within the abdomen. Also, clean peritoneal surfaces devoid of cancer cells are less likely to occur with sharp dissection or opposed to electrosurgical dissection. Lasermode electrosurgery leaves a margin of heat necrosis that is devoid of viable malignant cells. Not only does electroevaporation of tumor and normal tissue at the margins of resection minimize the likelihood of persistent disease, but also it minimizes blood loss. In the absence of lasermode electrosurgery, profuse bleeding from stripped peritoneal surfaces may occur during the intraperitoneal wash with chemotherapy.
Conversion of Peritoneal Surface to Invasive Malignancy by Surgery
Finally, extensive cytoreductions in the absence of perioperative intraperitoneal chemotherapy may actually a patient in the long-run rather than help them. Extensive removal of peritoneal surfaces without intraperitoneal chemotherapy will allow tumor cells to become implanted within a deeper layer of the abdomen and pelvis. This may contribute to obstruction of vital structures such as the ureter or common duct. Also, deep involvement of the pelvic sidewall and tissues along vascular structures will occur. If a surgeon attempts to treat peritoneal surface malignancy, he must become thoroughly familiar with the techniques of intraoperative chemotherapy, early postoperative chemotherapy, and induction intraperitoneal chemotherapy. Complete cytoreduction combined with aggressive perioperative intraperitoneal chemotherapy and proper patient selection are the three essential requirements of treatment for peritoneal surface malignancy.
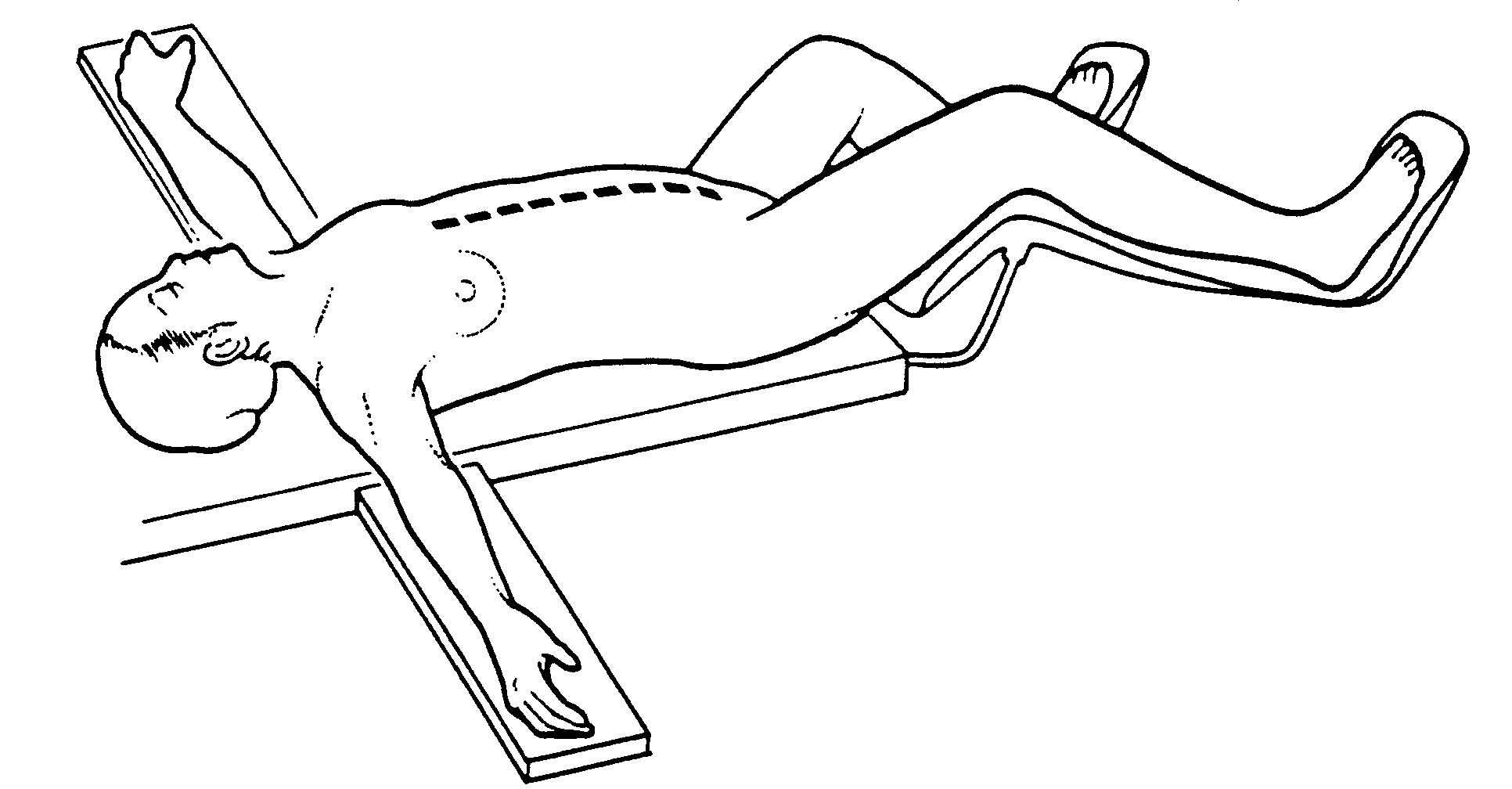
Position and Incision (Figure 1)
The patient is supine with the gluteal fold advanced to the end of the operating table to allow full access to the perineum during the surgical procedure. This lithotomy position is achieved with the legs extended in St. Mark’s leg holders (AMSCO, Erie, PA). The weight of the legs must be directed to the soles of the feet by positioning the foot rests so that minimal weight is on the calf muscle. Myonecrosis within the gastrocnemius muscle may occur unless the legs are protected properly. All surfaces of the St. Mark’s stirrups are protected by foam padding. The legs are surrounded by alternating-pressure boots (SCB Compression Boots, Kendall Co., Boston, MA). These should be operative before the start of anesthesia for maximal protection against venothrombosis. A heating/cooling blanket is placed over the chest and arms of the patient (Bair Hugger Upper Body Cover, Augustine Medical, Eden Prarie, MN 55344) and also beneath the torso (Cincinnati Sub-Zero, Cincinnati, OH).
Abdominal skin preparation is from mid-chest to mid-thigh. The external genitalia are prepared in the male and a vaginal preparation used in females. The Foley catheter is placed in position. A silastic 18-gauge nasogastric sump tube is placed within the stomach (Argyle Salem Sump Tube, Sherwood Medical, St. Louis, MO).
 |
Figure 1 |
Abdominal Exposure, Greater Omentectomy, and Splenectomy (Figure 2)
The abdomen is opened through a midline incision from xiphoid to pubis. Generous abdominal exposure is achieved through the use of a Thompson Self-Retaining Retractor (Thompson Surgical Instruments, Inc., Traverse City, MI). The standard tool used to dissect tumor on peritoneal surfaces from the normal tissues is a 3 mm ball-tipped electrosurgical handpiece (Valleylab, Boulder, CO). The ball-tipped instrument is placed at the interface of tumor and normal tissues. The focal point for further dissection is placed on strong traction. The electrosurgical generator is used on pure cut at high voltage. The 3 mm ball-tipped electrode is used cautiously for tumor removal on tubular structures, especially the ureters, small bowel, and colon. Dissection of parietal peritoneal surfaces presents less risk for heat necrosis and fistula formation.
Using ball-tipped electrosurgery on pure cut creates a large volume of plume because of the electroevaporation (carbonization) of tissue. To maintain visualization of the operative field and to preserve a smoke-free atmosphere, a smoke filtration unit is used (Stackhouse Inc., El Segunda, CA). The vacuum tip is maintained 2-3 inches from the field of dissection whenever electrosurgery is in use.
To free the mid-abdomen of a large volume of tumor, the greater omentectomy-splenectomy is performed. The greater omentum is elevated and then separated from the transverse colon using electrosurgery. This dissection continues beneath the peritoneum that covers the transverse mesocolon so as to expose the pancreas. The gastroepiploic vessels on the greater curvature of the stomach are clamped, ligated, and divided. Also, the short gastric vessels are transected. The mound of tumor that covers the spleen is identified. With traction on the spleen, the peritoneum anterior to the pancreas is stripped from the gland using electrosurgery. This freely exposes the splenic artery and vein at the tail of the pancreas. These vessels are ligated in continuity and proximally suture ligated. This allows the greater curvature of the stomach to be reflected to the right from the pylorus to the gastroesophageal junction.
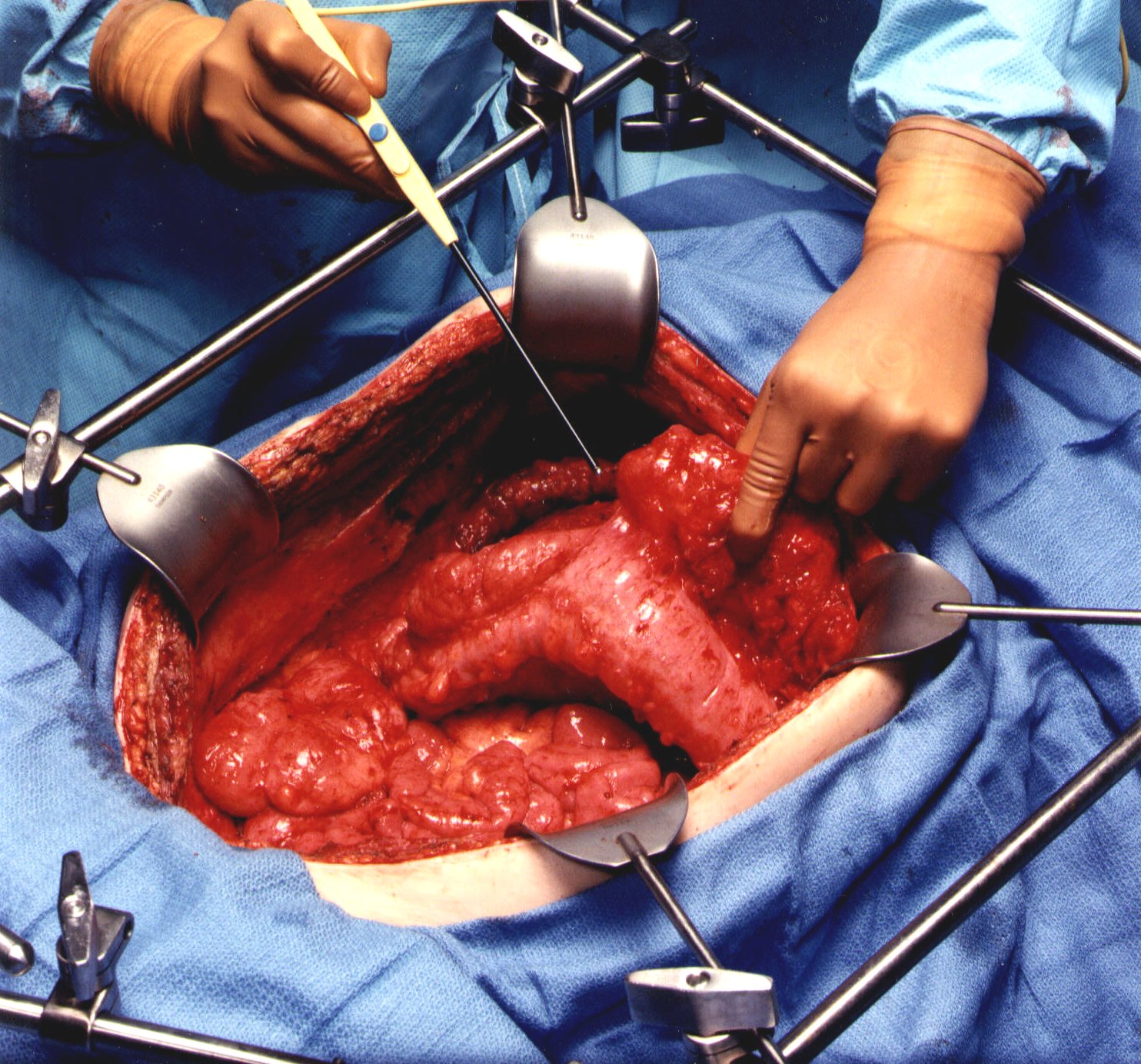 |
Figure 2 |
Peritoneal Stripping from Beneath the Left Hemidiaphragm (Figure 3)
To begin peritonectomy of the left upper quadrant, the peritoneum at the edge of the abdominal incision is stripped off the posterior rectus sheath. This allows strong traction to be exerted on the tumor specimen throughout the left upper quadrant and separation of surface tumor from all normal tissue in the left upper quadrant to the diaphragmatic muscle, the left adrenal gland, and the superior half of perirenal fat. The splenic flexure of the colon is severed from the left abdominal gutter and moved medially by dividing the peritoneum along Toldt’s line. The dissection beneath diaphragm muscle must be performed with ball-tipped electrosurgery, not by blunt dissection. Numerous blood vessels between the diaphragm muscle and its peritoneal surface must be electrocoagulated before their transection or unnecessary bleeding will occur as the divided blood vessel retracts into the muscle of the diaphragm. Tissues are transected using ball-tipped electrosurgery on pure cut, but all blood vessels are electrocoagulated before their division.
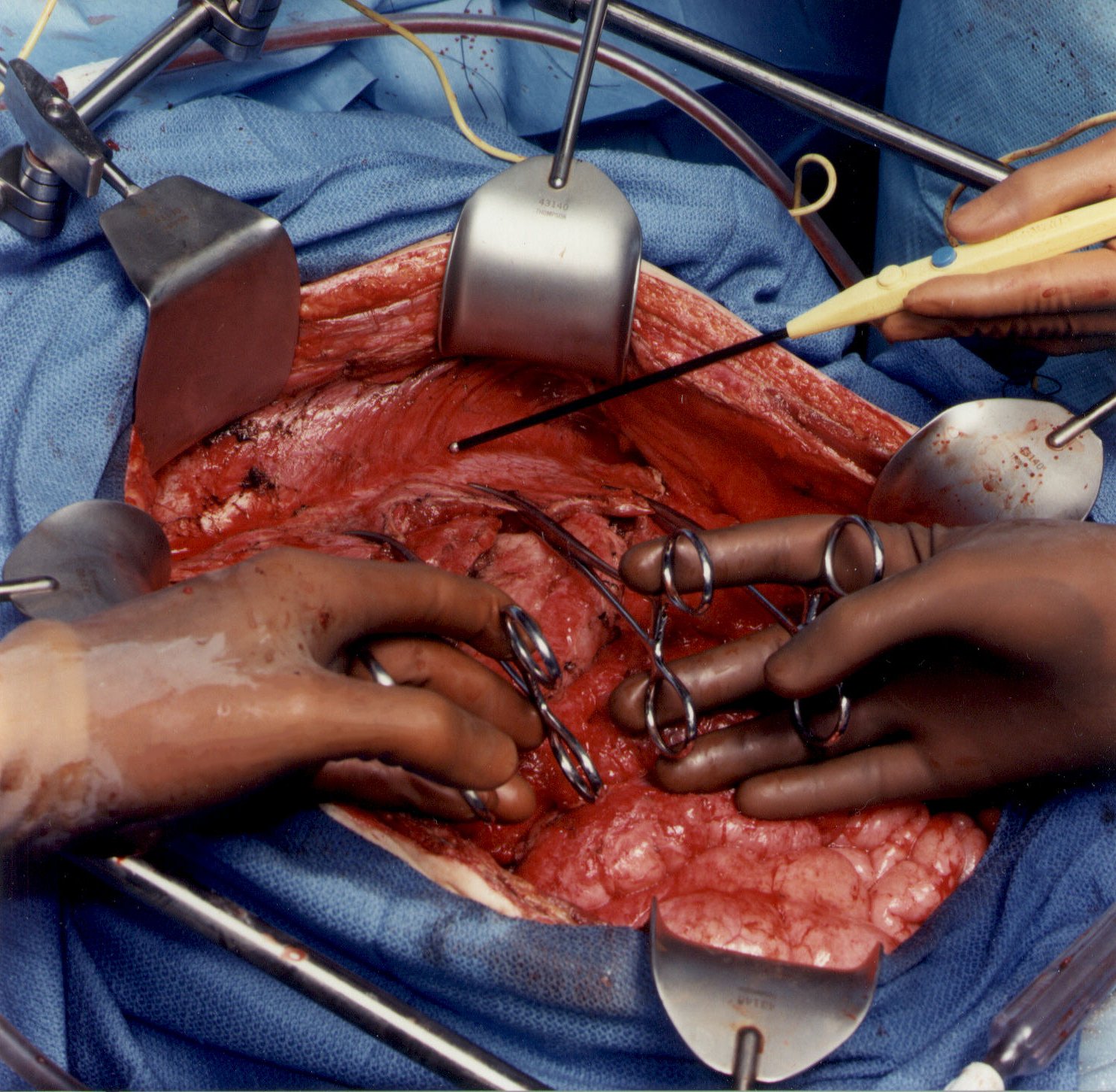 |
Figure 3 |
Left Subphrenic Peritonectomy Completed (Figure 4)
When the left upper quadrant peritonectomy is completed, the stomach may be reflected medially. Numerous branches of the gastroepiploic arteries that have been ligated are evident. The left adrenal gland, pancreas, and left Gerota’ fascia are visualized completely, as is the anterior peritoneal surface of the transverse mesocolon. The surgeon must avoid the left gastric artery and coronary vein to preserve the sole remaining vascular supply to the stomach.
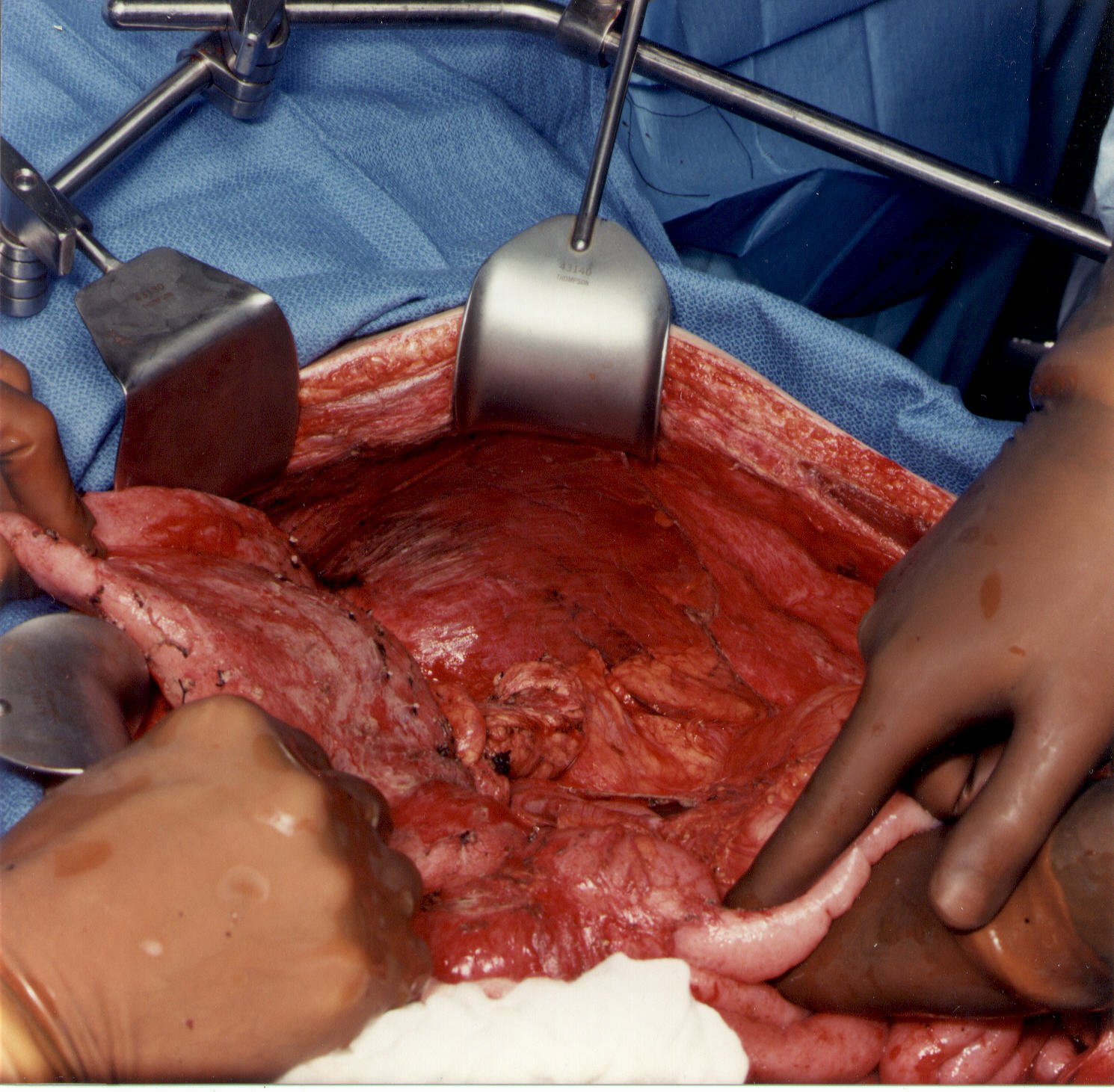 |
Figure 4 |
Peritoneal Stripping from Beneath the Right Hemidiaphragm (Figure 5)
Peritoneum is stripped from the right posterior rectus sheath to begin the peritonectomy in the right upper quadrant of the abdomen. Strong traction on the specimen is used to elevate the hemidiaphragm into the operative field. Again, ball-tipped electrosurgery on pure cut is used to dissect at the interface of tumor and normal tissue. Coagulation current is used to divide the blood vessels as they are encountered and before they bleed.
The stripping of tumor from the undersurface of the diaphragm continues until the bare area of the liver is encountered. At that point, tumor on the superior surface of the liver is electroevaporated until the liver surface is cleared. With ball-tipped electrosurgical dissection, a thick layer of tumor may be lifted off the dome of the liver by moving beneath Glisson’s capsule. Isolated patches of tumor on the liver surface are electroevaporated with the distal 2 cm of the ball tip bent and stripped of insulation ("hockey-stick" configuration). Ball-tipped electrosurgery is also used to extirpate tumor from attachments of the falciform ligament and round ligament.
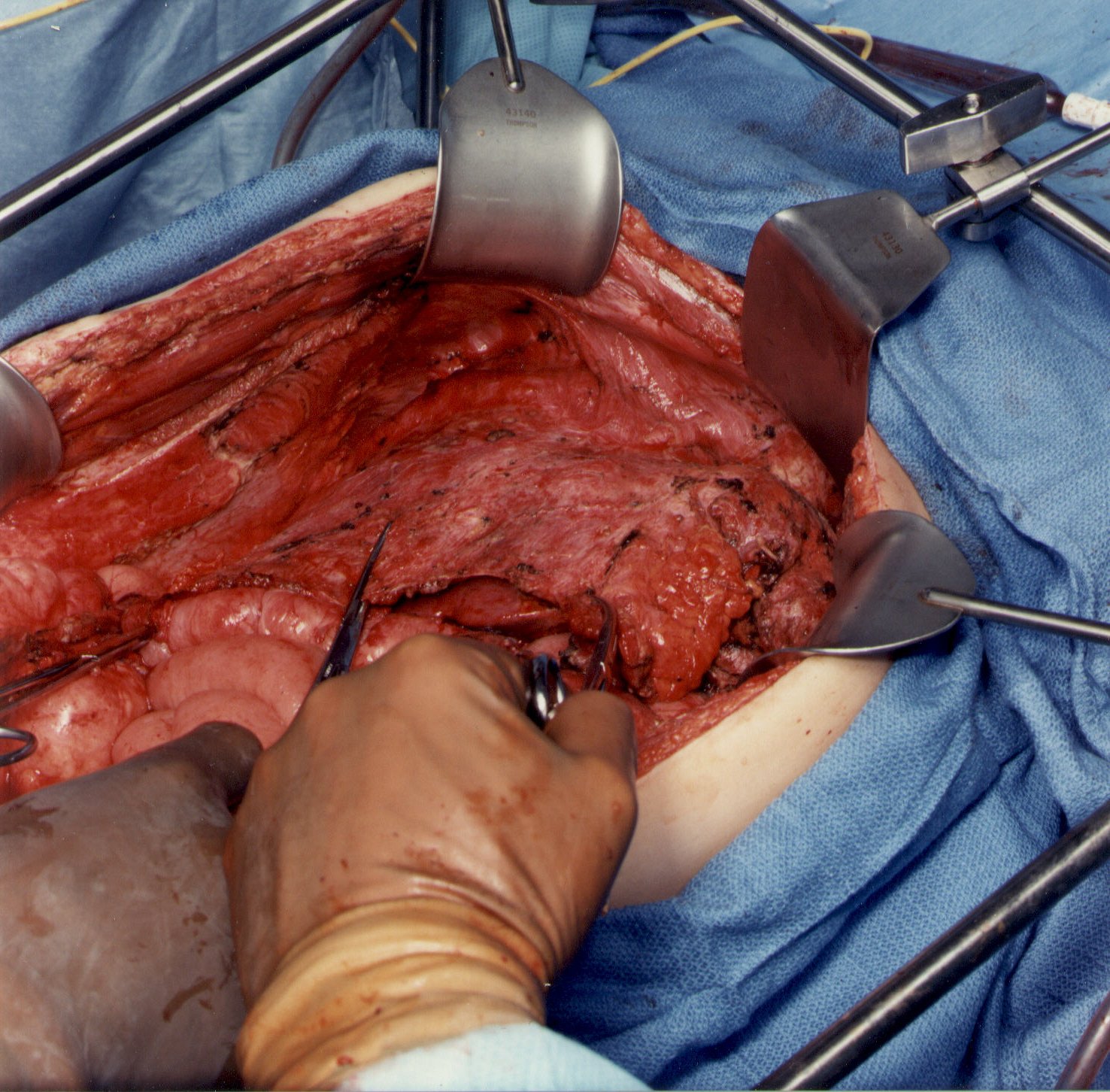 |
Figure 5 |
Stripping of Tumor from Beneath the Right
Hemidiaphragm, from Right Subhepatic Space, and from the
Surface of the Liver (Figure 6)
Tumor from beneath the right hemidiaphragm, from the right subhepatic space, and from the surface of the liver forms an envelope as it is removed en bloc. The dissection is greatly facilitated if the tumor specimen can be maintained intact. The dissection continues laterally on the right to encounter the perirenal fat covering the right kidney. Also, the right adrenal gland is visualized and carefully avoided as tumor is stripped from the right subhepatic space. Care is taken not to traumatize the vena cava or to disrupt the caudate lobe veins that pass between the vena cava and segment 1 of the liver.
 |
Figure 6 |
Completed Right Subphrenic Peritonectomy (Figure 7)
With strong, upward traction on the right coastal margin by the self-retaining retractor and medial displacement of the right liver, one can visualize the completed right subphrenic peritonectomy. The anterior branches of the phrenic artery and vein on the hemidiaphragm are seen and have been preserved. The right hepatic vein and the vena cava below have been exposed. The right subhepatic space including the right adrenal gland and perirenal fat covering the right kidney constitutes the base of the dissection.
Frequently, tumor is densely adherent to the tendinous central portion of the left or right hemidiaphragm. If this occurs, the tissue infiltrated by tumor must be resected. This usually requires an elliptical excision of a portion of the hemidiaphragm on either the right or the left. The defect in the diaphragm is closed with interrupted sutures after the intraoperative chemotherapy is completed.
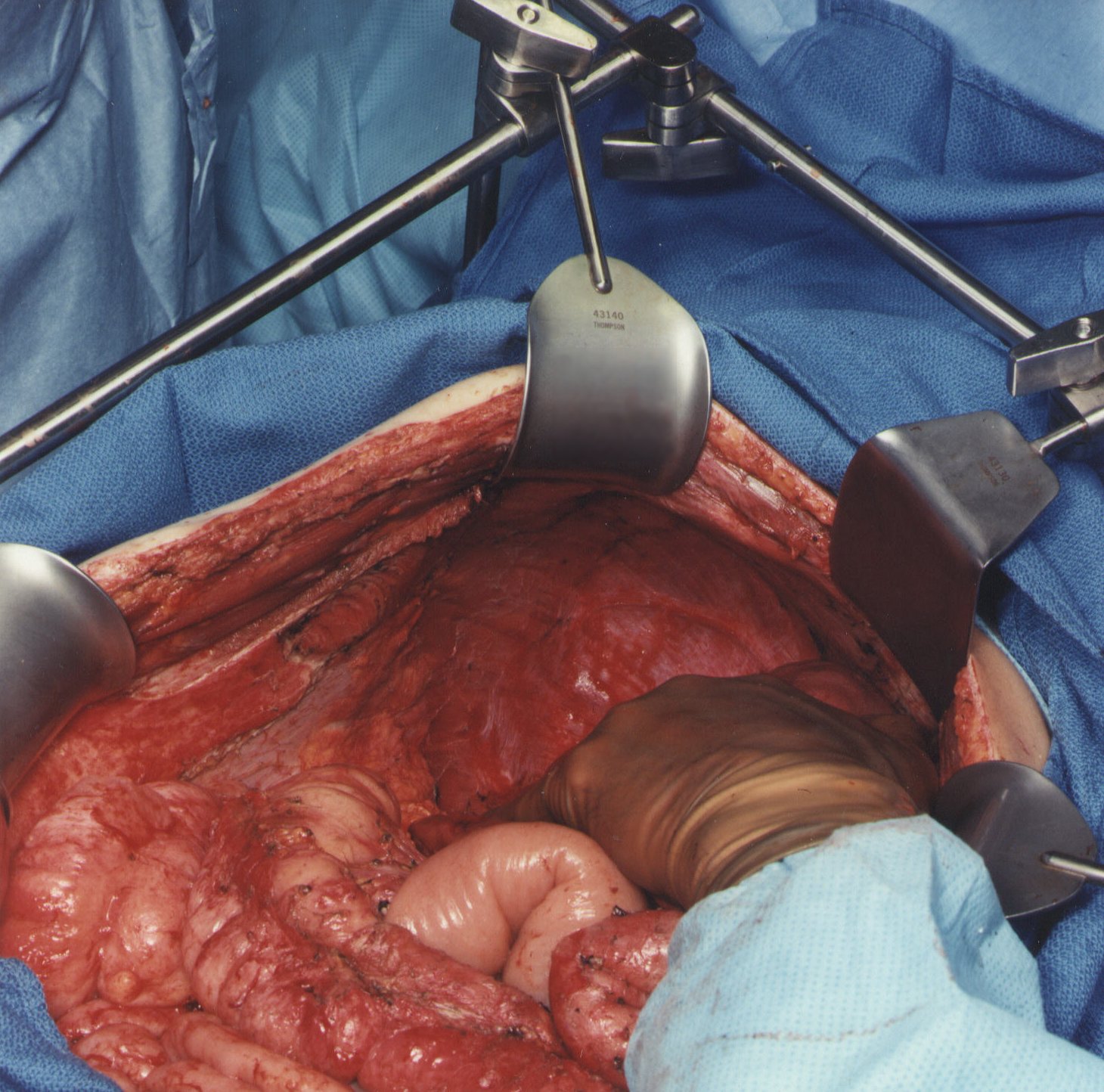 |
Figure 7 |
Lesser Omentectomy and Cholecystectomy with Stripping of the Porta Hepatis (Figure 8)
The gallbladder is removed in a routine fashion from its fundus toward the cystic artery and cystic duct. These structures are ligated and divided. The right lateral portion of the hepatoduodenal ligament that covers the porta hepatis is characteristically heavily layered with tumor. Using strong traction, the cancerous tissue that coats the porta hepatis is bluntly stripped from the base of the gallbladder bed toward the duodenum. The right gastric artery going to the lesser omental arcade is preserved. To continue resection of the lesser omentum, one dissects through the gastrohepatic fissure that divides liver segments 2, 3, and 4 from segment 1. Ball-tipped electrosurgery is used to electroevaporate tumor from the anterior surface of the left caudate process. Care is taken not to traumatize the anterior surface of the caudate process, for this can result in excessive and needless blood loss. The segmental blood supply to the caudate lobe is located on the anterior surface of this segment of the liver, and hemorrhage may occur with only superficial trauma. Also, a replaced left hepatic artery may arise from the left gastric artery and cross through the hepatogastric fissure.
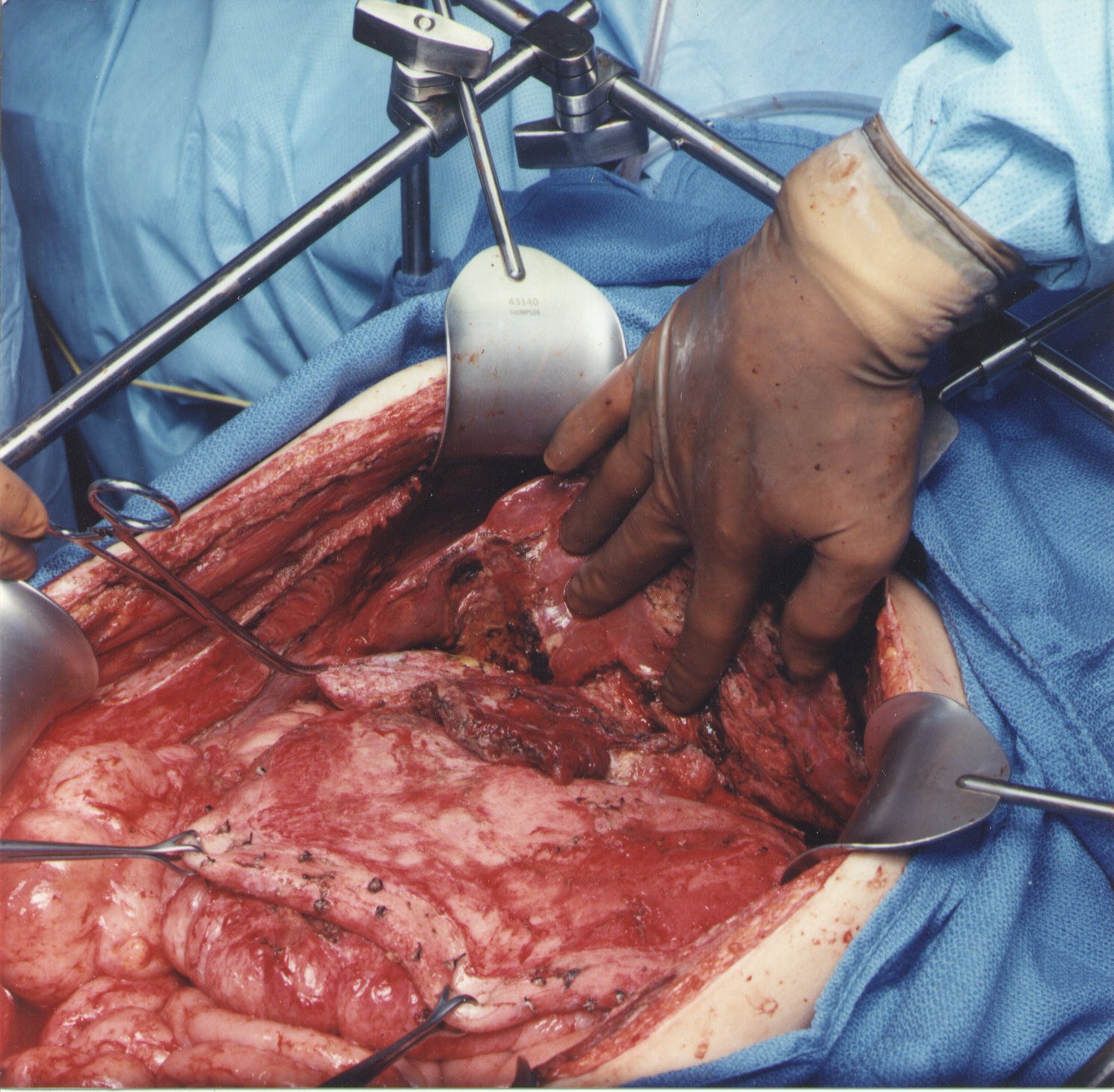 |
Figure 8 |
Stripping of the Omental Bursa (Figure 9)
As one clears the left part of liver segment one of tumor, the vena cava is visualized directly beneath. To begin to strip the omental bursa, strong traction is maintained on the tumor and ball-tipped electrosurgery is used to divide the fibrous tissue between liver segment I and the vena cava. The phrenoesophageal ligament is incised so that peritoneum can be stripped away from the crus of the right hemidiaphragm. The common hepatic artery and the left gastric artery are skeletonized and lymph nodes in this region avoided. The cephalad and caudad branching of the left gastric artery and the coronary vein are identified and avoided. Dissection of lesser omental fat by compressing tissue between the thumb and index finger helps identify the major branches of the left gastric artery. Omental fat not involved by tumor is preserved to ensure adequate blood supply to the stomach. At least two major branches of the left gastric artery to the lesser curvature of the stomach are required to provide blood supply to the stomach.
The surgeon dissects in a clockwise direction along the lesser curvature of the stomach, attempting to preserve the arcade. Care is taken to preserve as much omental fat as possible; only tumor tissue is removed. One attempts to spare the anterior vagus nerve going toward the antrum of the stomach.
Sometimes, a pyloroplasty or gastrojejunostomy must be performed if the vagus nerve is divided. As a result of the anterior vagotomy and in the absence of a gastric drainage procedure, gastric stasis may occur.
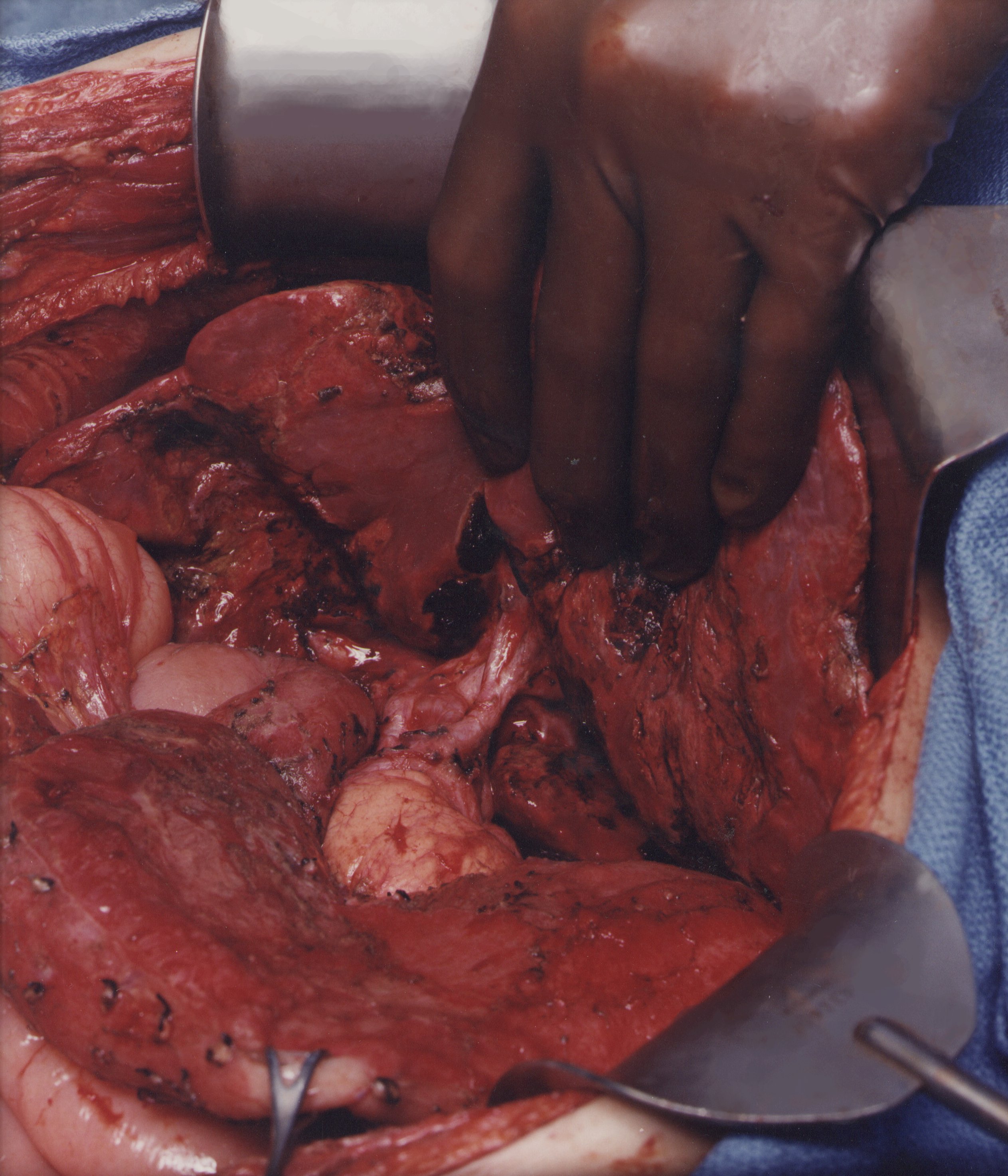 |
Figure 9 |
Complete Pelvic Peritonectomy (Figure10)
The tumor-bearing peritoneum is stripped from the posterior surface of the lower abdominal incision, exposing the rectus muscle. The muscular surface of the bladder is seen, as ball-tipped electrosurgery strips peritoneum and preperitoneal fat from this structure. The urachus must be divided and is then elevated on a clamp as the leading point for this dissection. In the female, the round ligaments are divided as they enter the internal inguinal ring.
The peritoneal incision around the pelvis is completed by dividing the peritoneum along the pelvic brim. The right and left ureters are identified and preserved. In women, the right and left ovarian veins are ligated at the level of the lower pole of the kidney and divided. A linear stapler is used to divide the sigmoid colon just above the limits of the pelvic tumor. The vascular supply of the distal portion of the bowel is traced back to its origin on the aorta. The inferior mesenteric artery is suture ligated and divided. This allows one to pack all the viscera, including the proximal sigmoid colon, in the upper abdomen.
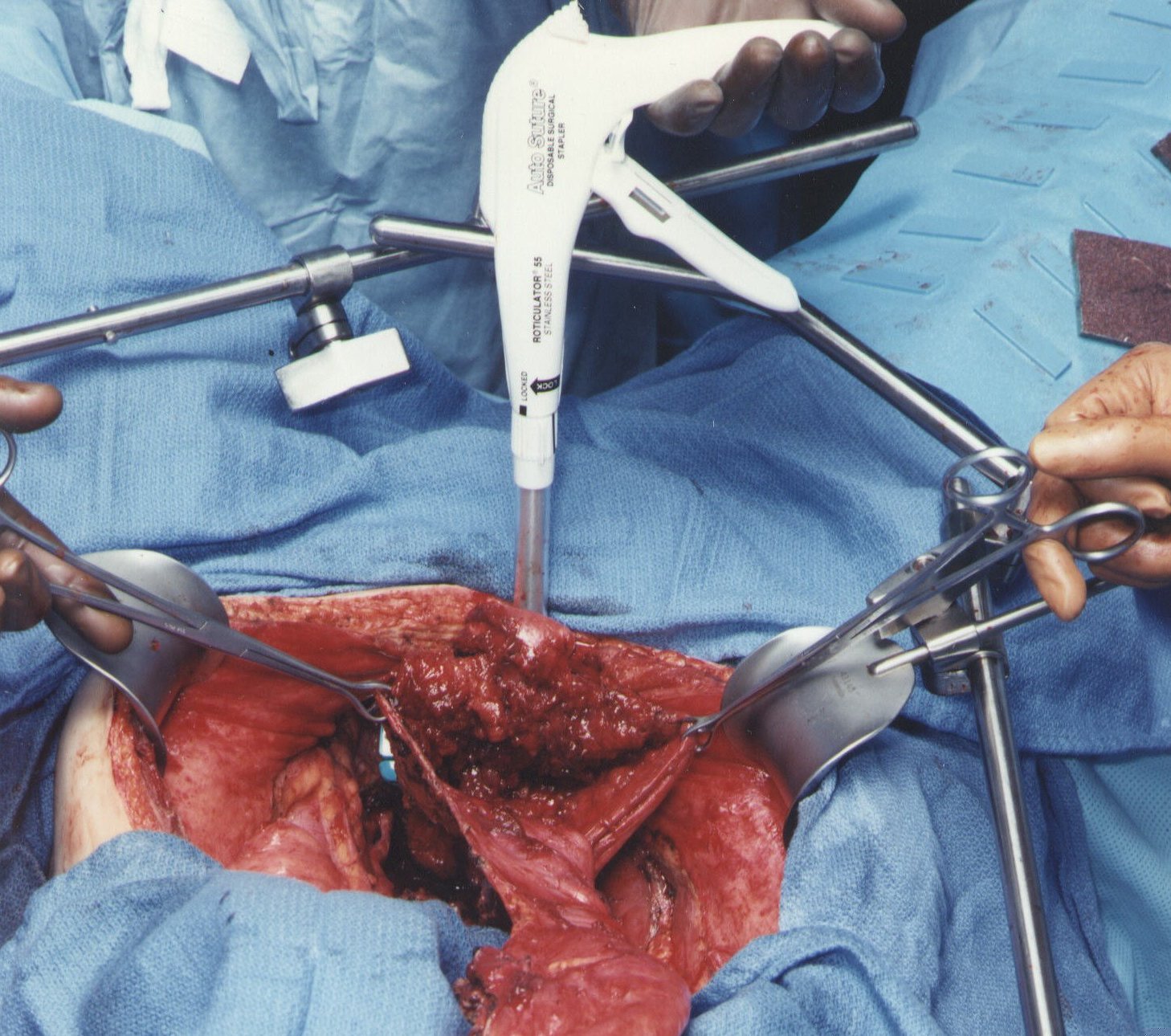 |
Figure 10 |
Resection of Rectosigmoid Colon and Cul-de-Sac of Douglas. (Figure11)
Ball-tipped electrosurgery is used to dissect at the limits of the mesorectum. The surgeon works in a centripetal fashion. Extraperitoneal ligation of the uterine arteries is performed just above the ureter and close to the base of the bladder. In women, the bladder is moved gently off the cervix and the vagina is entered. The vaginal cuff anterior and posterior to the cervix is transected using ball-tipped electrosurgery, and the rectovaginal septum is entered. Ball-tipped electrosurgery is used to divide the perirectal fat beneath the peritoneal reflection. This ensures that all tumors that occupy the cul-de-sac are removed intact with the specimen. The rectal musculature is skeletonized using ball-tipped electrosurgery. A roticulator stapler (Autosuture, Norwalk, CT) is used to close off the rectal stump closed and the rectum is sharply divided above the stapler with a scissor.
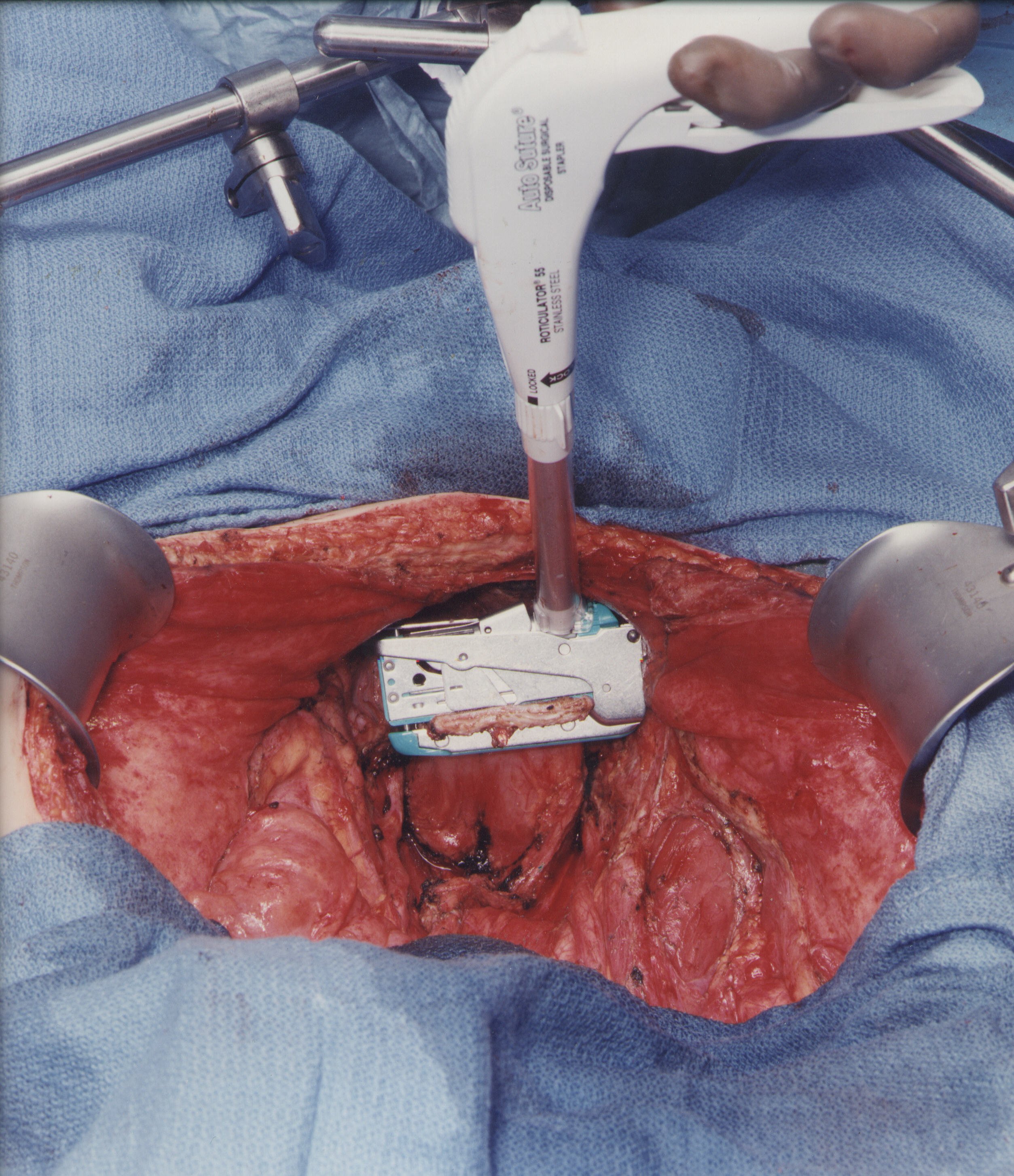 |
Figure 11 |
Vaginal Closure and Low Colorectal Anastomosis (Figure 12)
One of the few suture repairs performed prior to the intraoperative chemotherapy is the closure of the vaginal cuff. If one fails to close the vaginal cuff, chemotherapy containing fluid will leak from the vagina. The circular stapled colorectal anastomosis occurs after intraoperative chemotherapy. A circular stapling device is passed into the rectum, and the trochar penetrates the staple line. A monofilament suture placed in a purse-string fashion is used to secure the staple anvil in the proximal sigmoid colon. The body of the circular stapler and anvil are mated and the stapler is activated to complete the low colorectal anastomosis (Intraluminal Stapler 33, Ethicon, Sommerville, NJ).
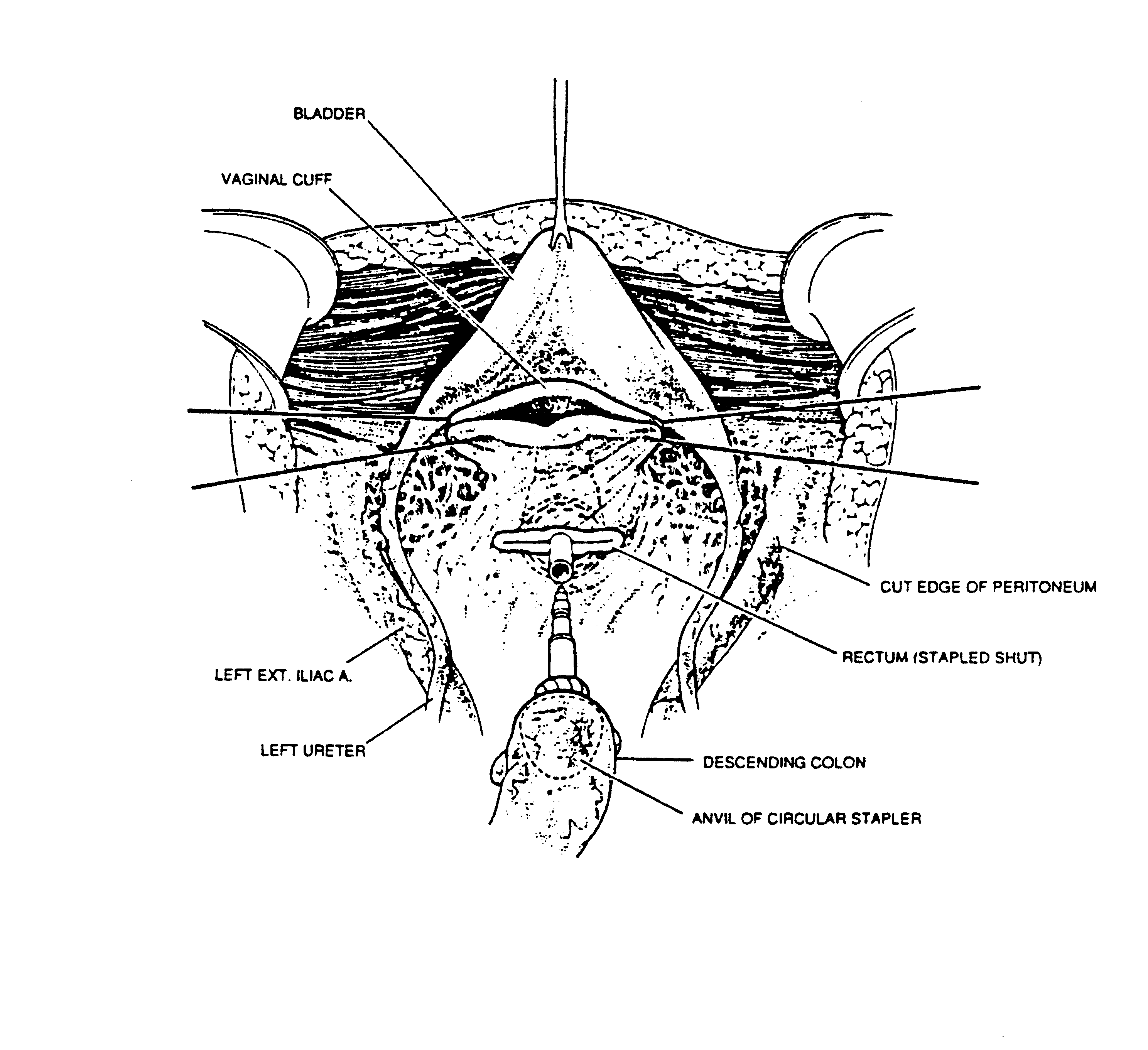 |
Figure 12 |
Left Colon Mobilization for a Tension Free Low Colorectal Anastomosis (Figure 13)
A requirement for a complication free low colorectal anastomosis is absence of tension on the staple line. Adequate mobilization of the entire left colon is needed, and several steps may be required to accomplish this. The inferior mesenteric artery is ligated on the aorta, and then its individual branches are resected as they arise from this vascular trunk. This is the Y to V transition that keeps the intermediate arcade intact. The inferior mesenteric vein is divided as it courses around the duodenum. The mesentery of the transverse colon and splenic flexure are completely elevated from the perirenal fat surrounding the left kidney. Taking care to avoid the left ureter, the surgeon divides the left colon mesentery from all its attachments. These maneuvers allow the junction of the sigmoid and descending colon to reach to the low rectum or anus for a tension-free anastomosis. Redundant descending colon should fall into the hollow of the sacrum.
To monitor the stapled colorectal anastomosis, the proximal and distal tissue rings are examined for completeness. Air is insufflated into the rectum with a water-filled pelvis to check for an airtight circle of staples. Two hands should easily pass beneath the sigmoid colon to ensure there is no tension on the stapled anastomosis. A rectal examination is done to check for staple-line bleeding at the anastomosis.
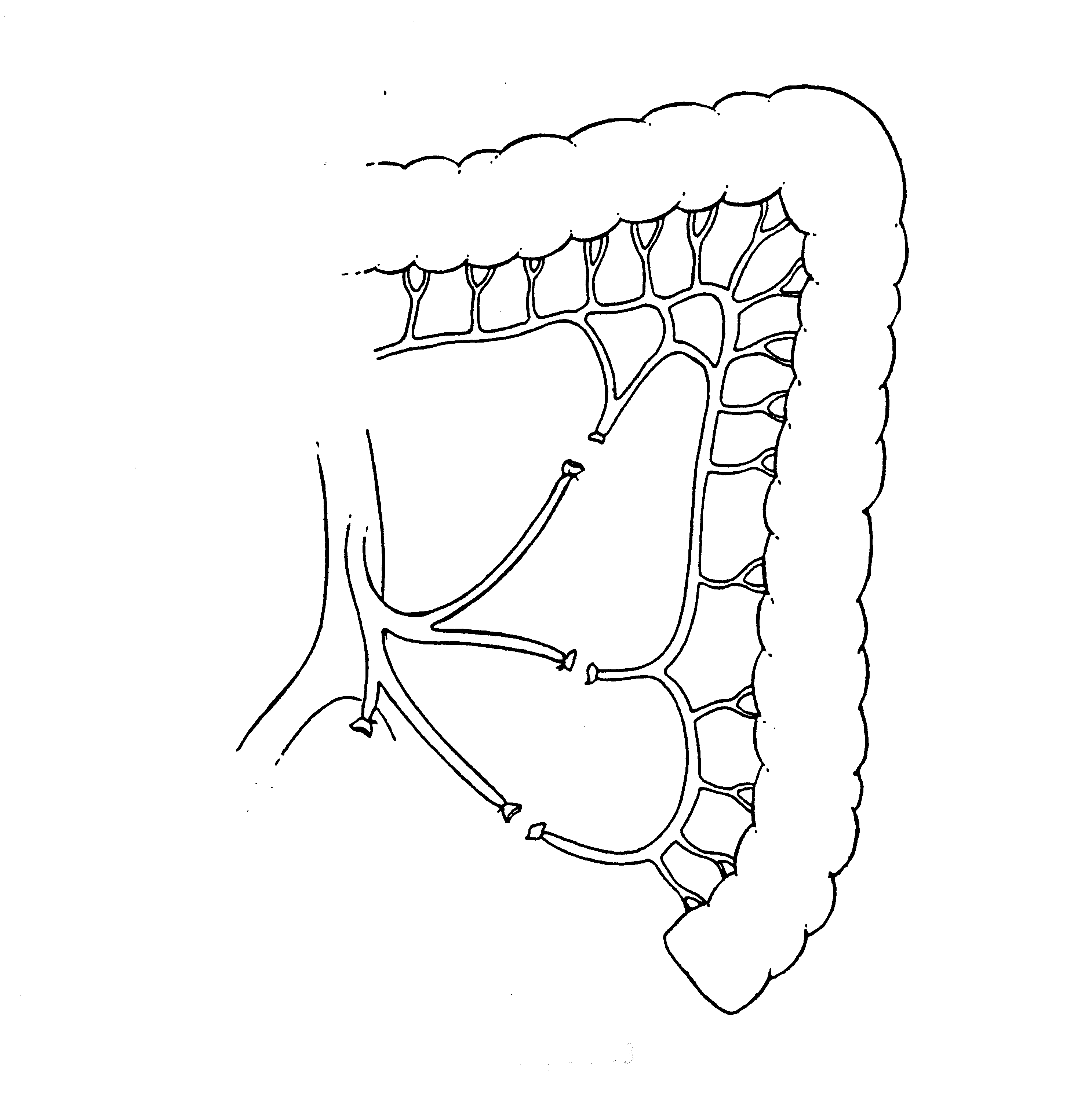 |
Figure 13 |
Antrectomy and Gastric Reconstruction (Figure 14)
The gastric antrum, along with other intraabdominal structures that have restricted peristalsis, may be surrounded so densely by tumor that resection rather than peritoneal stripped is required for complete tumor removal. The right gastric artery is divided and the first portion of the duodenum is separated from the pancreas. A stapler (Ethicon PLC75, Cincinnati, OH) is used to close off and transact the duodenum just below the last visible evidence of tumor. Similarly, a stapler (Ethicon TA90, Cincinnati, OH) divides the stomach proximally above the tumor. The duodenal and gastric staple lines are inverted with interrupted sutures after intraoperative chemotherapy has been completed. A side-to-side gastrojejunostomy is performed after the intraoperative chemotherapy is complete.
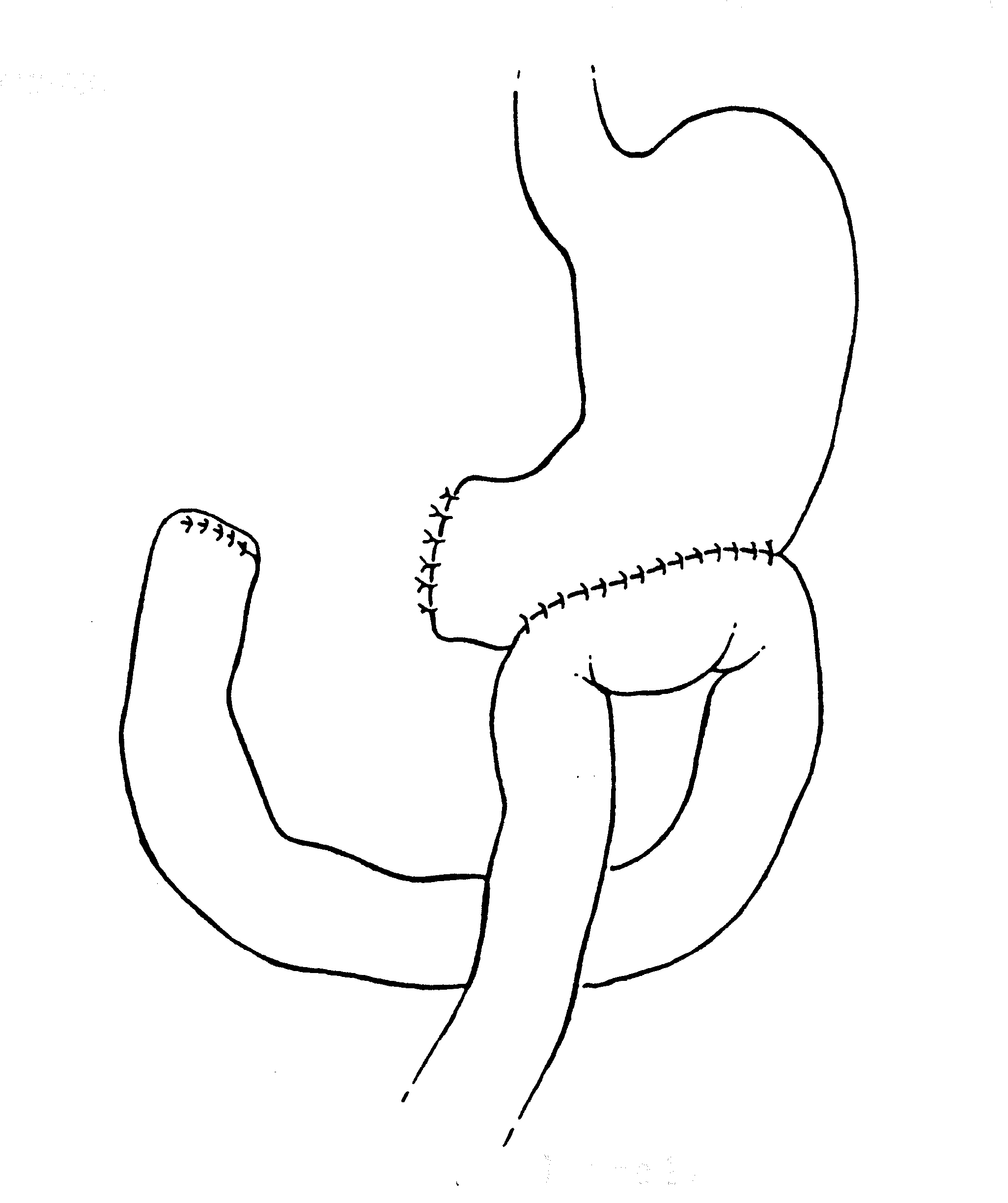 |
Figure 14 |
Total Gastrectomy with Staged Reconstruction (Figure 15)
In approximately 10% of patients with pseudomyxoma peritoneal, a total gastrectomy will be needed to clear the left upper quadrant of mucinous tumor. In most instances, this indicates that the tumor is a more aggressive type, usually called pseudomyxoma/carcinoma hybrid. Alternatively, the patient may have had many prior surgical procedures with prior extensive dissection in the left upper quadrant.
To perform the gastrectomy, the esophagus is closed off with a linear stapler (Ethicon TA30, Cincinnati, OH) and then transected. The left gastric artery is ligated and suture ligated. Final attachments of the stomach to the superior portion of the head of the pancreas are divided using ball-tipped electrosurgery. Great care is taken to not damage the anterior surface of the pancreas.
To reconstruct the gastrointestinal tract after gastrectomy that is part of a complete cytoreduction, a duodenal exclusion operation is performed. This protects the esophagojejunal anastomosis. Approximately 20 cm below the Ligament of Treitz, a portion of jejunum is transected with a linear stapler and brought in a retrocolic fashion up to the esophagus. The esophageal staple line is removed and a purse-string suture is used to secure the anvil of a circular stapler in the distal esophagus (Ethicon ILS29, Cincinnati, OH). The staple line closing the proximal jejunum is removed and the stapler is passed approximately 5 cm into the jejunum and then out through the jejunal wall. It is mated with the anvil within the esophagus, and the staple line is completed. The proximal jejunum is stapled off, and then the staple line inverted with interrupted sutures. This reconstruction is performed after the intraoperative chemotherapy is complete.
The portion of jejunum proximal to the linear staple line is now brought out in the left upper quadrant as an end ostomy in order to divert all bile and digestive enzymes from the gastrointestinal tract. This diverting jejunostomy is closed between 6 and 9 months postoperatively as part of a second-look procedure.
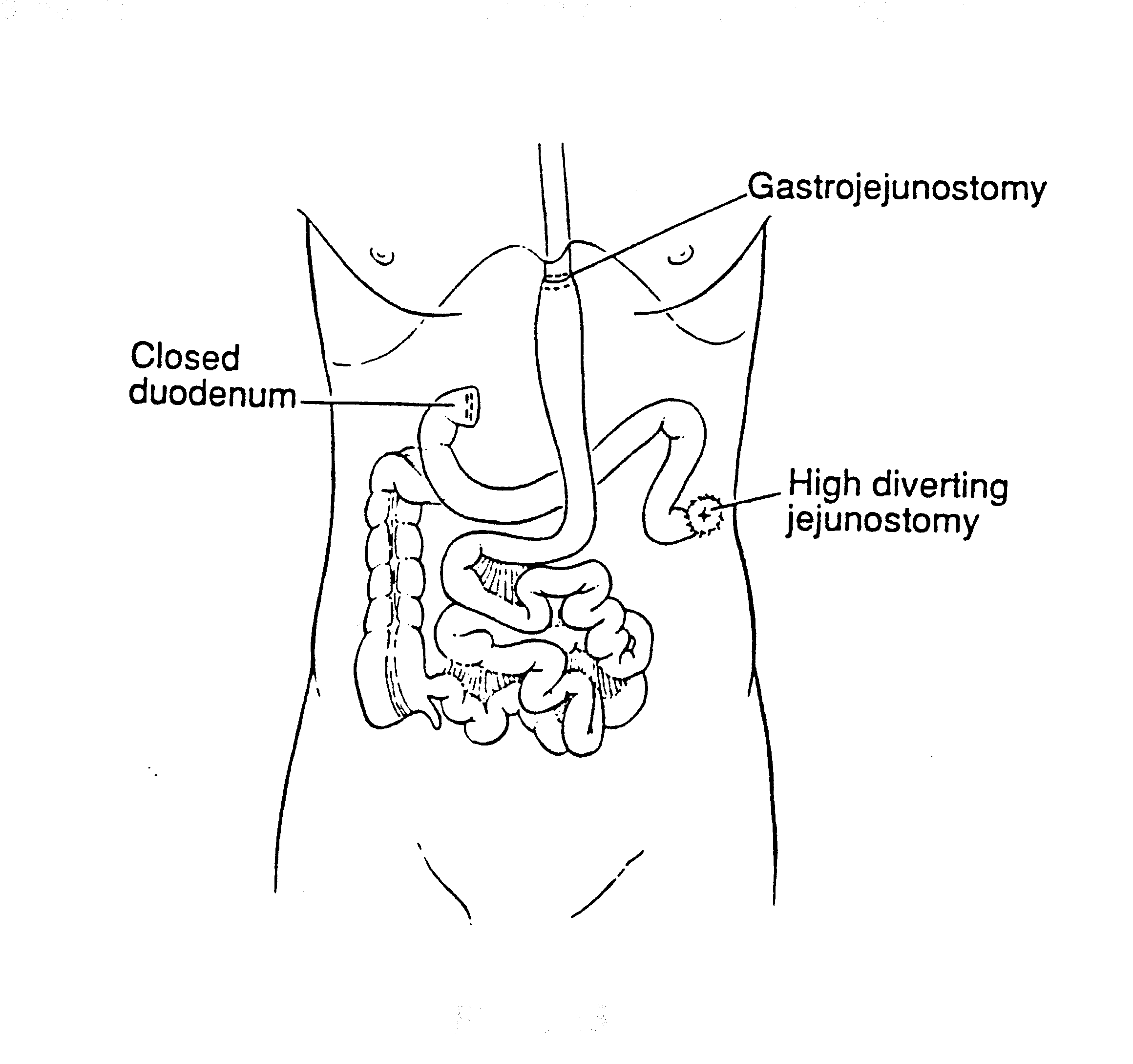 |
Figure 15 |
Tubes and Drains Required for Intraoperative and Early Postoperative Intraperitoneal Chemotherapy (Figure 16)
Four closed-suction drains are placed in the dependent portions of the abdomen. This includes one in the right subhepatic space, one in the left subdiaphragmatic space, and two in the pelvis. A Tenckhoff catheter (Quinton curled peritoneal catheter, Quinton, Inc., Seattle, WA) is placed through the abdominal wall and positioned within the abdomen at the site that is thought to be the area of greatest risk for recurrence. All transabdominal drains and tubes are secured to the skin in a watertight fashion with a purse-string suture. Temperature probes are placed at the inflow (Tenckhoff catheter) and at a remote site. They are removed after the intraoperative chemotherapy is completed. Right angle thoracostomy tubes (Deknatel, Floral Park, NY) are inserted on both the right and left to prevent abdominal fluid from accumulating in the chest as a result of the subphrenic peritonectomy.
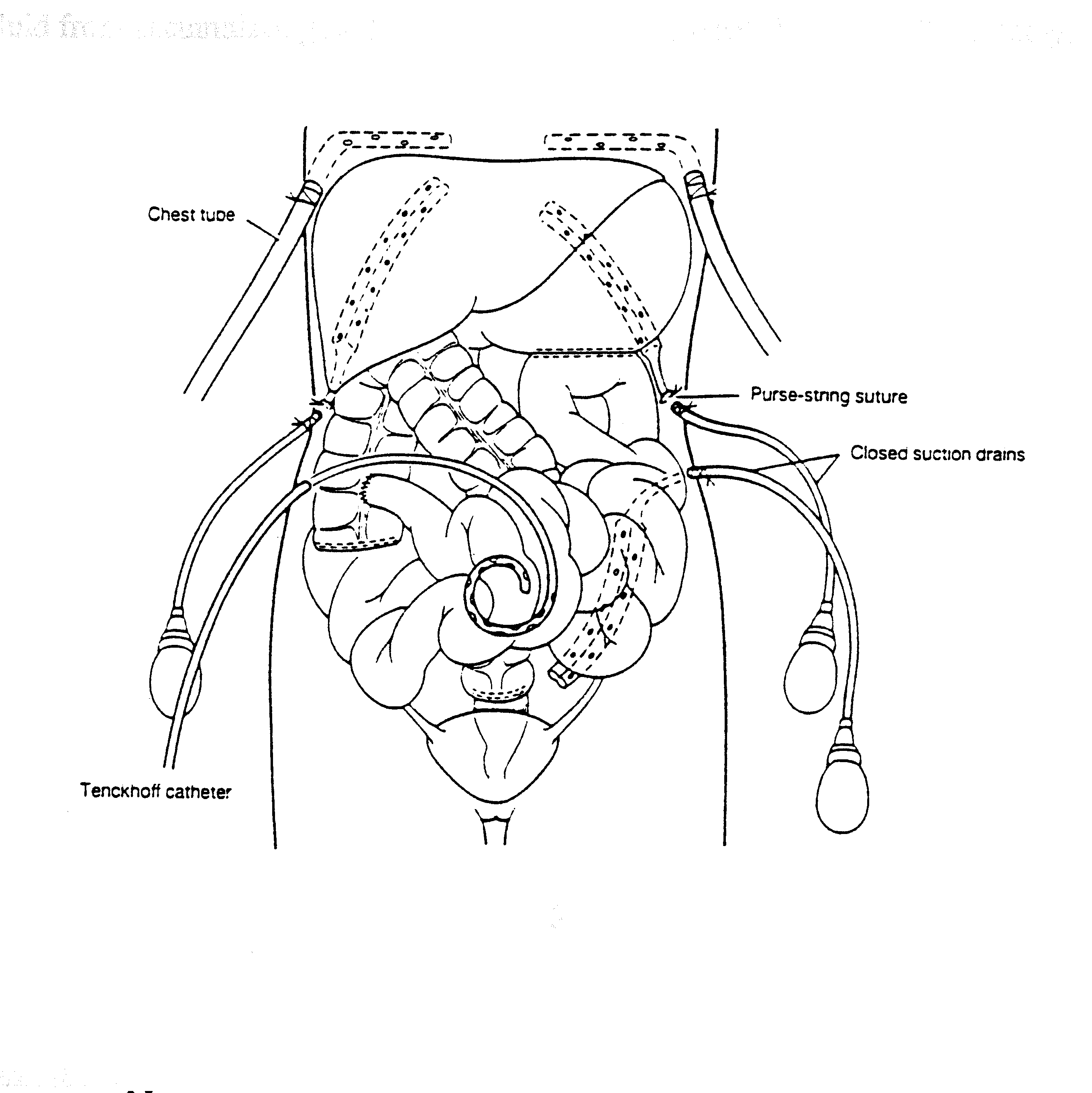 |
Figure 16 |
B. Intraperitoneal Chemotherapy
Conceptual Changes with Intraperitoneal Chemotherapy
Changes in the use of chemotherapy in patients with peritoneal carcinomatosis, peritoneal sarcomatosis, and peritoneal mesothelioma have occurred and shown favorable results of treatment. A change in ROUTE of drug administration has occurred. Chemotherapy is given intraperitoneally, or by combined intraperitoneal and intravenous routes. In this new strategy, intravenous chemotherapy alone is rarely indicated. Also, a change in TIMING has occurred in that chemotherapy begins in the operating room and may be continued for the first five postoperative days. Third, a change in SELECTION criteria for treatment of cancer has occurred. With the nonaggressive peritoneal surface malignancies as an exception. The lesion size of peritoneal implants is of crucial importance. Only patients with small intraperitoneal tumor nodules that have a limited distribution within the abdomen and pelvis are likely to show prolonged benefit. Meticulous cytoreductive surgery is necessary prior to the intraperitoneal chemotherapy instillation. Aggressive treatment strategies for an advanced and invasive intraperitoneal malignancy will not produce long-term benefits, and is often the cause of excessive morbidity or mortality. The initiation of treatments for peritoneal surface malignancy must occur as early as is possible in the natural history of these diseases in order to achieve the greatest benefits. The greatest change that now needs to occur with peritoneal surface malignancy is a change in oncologists’ attitudes toward these diseases. They may be cured with early application of combined treatments.
Background
Most cancers that occur within the abdomen or pelvis will disseminate by 3 different routes. These are hematogenous metastases, lymphatic metastases, and spread through the peritoneal space to surfaces within the abdomen and pelvis. In a substantial number of patients with abdominal or pelvic malignancy, surgical treatment failure is isolated to the resection site or to peritoneal surfaces. This leads to a hypothesis that suggests that the elimination of peritoneal surface spread may have an impact on the survival of these cancer patients, and that a leading cause of death and suffering in patients with these malignancies is progression of peritoneal surface disease. Prior to the use of cytoreductive surgery and intraperitoneal chemotherapy these conditions were uniformly fatal, eventually resulting in intestinal obstruction. Occasionally patients with low grade malignancies such as pseudomyxoma peritonei survived for several years, but all end results reportings have shown fatal outcomes.
Current technology for the administration of intraperitoneal chemotherapy demands that it be used as an integral part of the surgical procedure. "Surgically directed chemotherapy" involves several crucial technological modifications of chemotherapy administration. First, an intraperitoneal rather than an intravenous route for chemotherapy is used. The intraperitoneal route, when properly utilized, will allow uniform distribution of a high concentration of anticancer therapy at the site of the malignancy. This is achieved by the surgeon intraoperatively manipulating the intestinal contents to uniformly distribute the chemotherapy. In the early postoperative period, the patient’s position is repeatedly changed to assist gravity in maintaining an optimal chemotherapy distribution.
Secondly, the chemotherapy administration is timed so that all of the malignancy, except for microscopic residual disease, will have been removed prior to the chemotherapy treatments. This means that the limited penetration of chemotherapy into tissues, which is approximately 1 mm, will be adequate to eradicate all tumor cells. Also, the chemotherapy will be used prior to the construction of any anastomosis. This means that suture line recurrences should also be eliminated. Finally, since all adhesions have been taken down, there will be no surfaces in the abdomen or pelvis excluded by scar tissue from contact with chemotherapy solutions.
From all theoretical considerations, cytoreductive surgery and intraperitoneal chemotherapy must be used as early in the natural history of the cancer as is possible. No longer can the clinician wait for the patient with peritoneal carcinomatosis to become symptomatic to begin treatments. Protocols to prevent iatrogenic peritoneal surface spread that may occur as a result of the resection of a primary gastrointestinal malignancy must be considered. The treatment of patients with an invasive malignancy that has a wide distribution of a large mass of cancer will not produce long-term benefits. As oncologists accept that peritoneal surface malignancy can be cured, they will initiate aggressive treatments in a timely fashion.
Peritoneal-Plasma Barrier
Intraperitoneal chemotherapy gives high response rates within the abdomen because the "peritoneal plasma barrier" provides dose intensive therapy.(7) Figure 17 shows that large molecular weight substances, such as mitomycin C, are confined to the abdominal cavity for long time periods.(8) This means that the exposure of peritoneal surfaces to pharmacologically active molecules can be increased considerably by giving the drugs via the intraperitoneal route rather than the intravenous route.
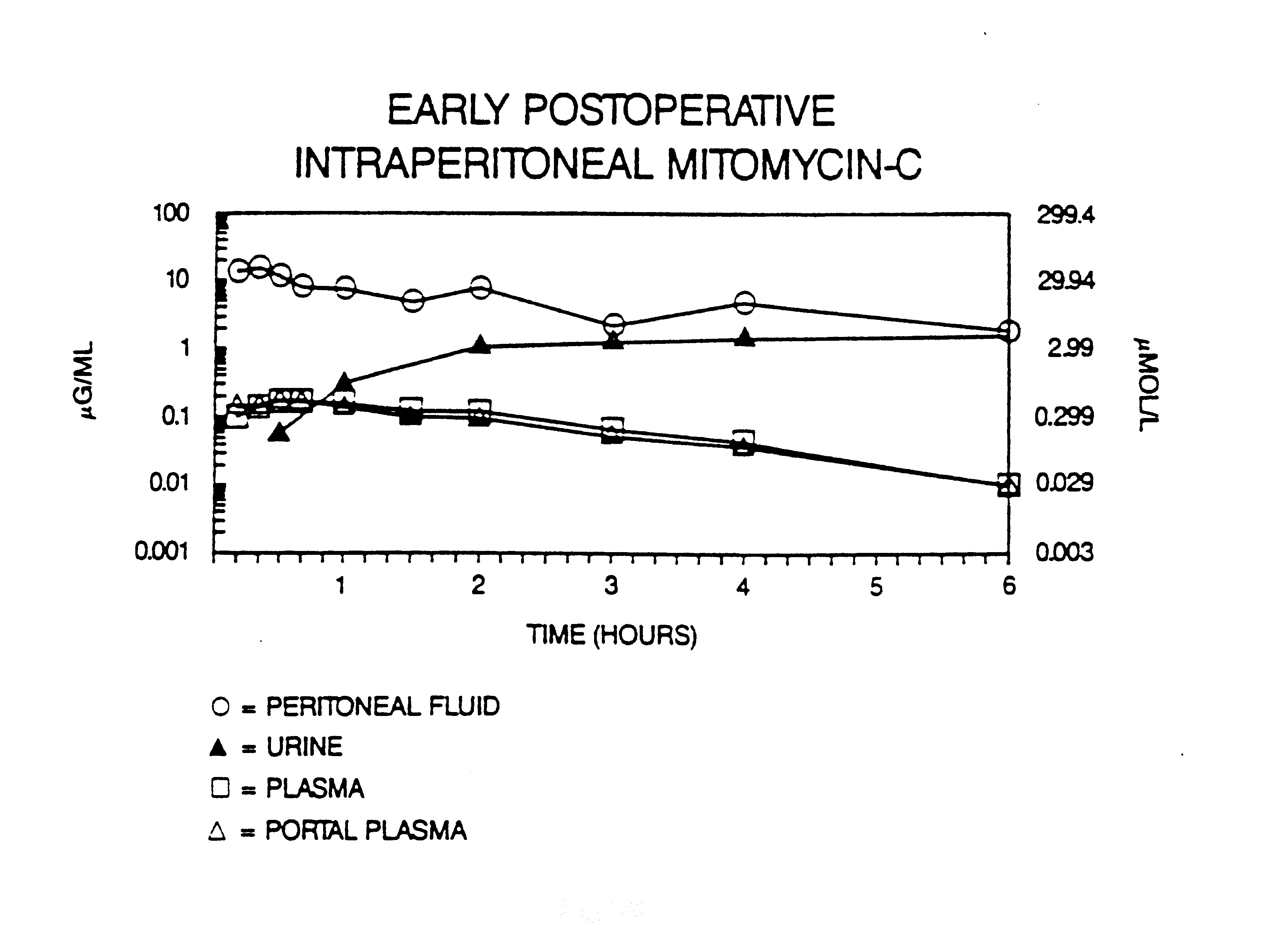 |
Figure 17 |
For the chemotherapy agents used to treat peritoneal carcinomatosis or peritoneal sarcomatosis, the area under the curve ratios of intraperitoneal to intravenous exposure are favorable. Table 1 presents the area under the curve (intraperitoneal/intravenous) for the drugs in routine clinical use in patients with peritoneal seeding. In our studies, these include 5-fluorouracil, mitomycin C, doxorubicin, cisplatin, taxol and gemcitabine.
Area
under the curve ratios of peritoneal surface exposure to
systemic exposure |
||
| Drug | Molecular Weight | Area Under the Curve Ratio |
| 5-Fluorouracil | 130 | 250 |
| Mitomycin C | 334 | 75 |
| Doxorubicin | 544 | 500 |
| Cisplatin | 300 | 20 |
| Taxol | 808 | 1000 |
| Gemcitabine | 263 | 50 |
Table 1 |
||
One should not assume that the intraperitoneal administration of chemotherapy eliminates their systemic toxicities. Although the drugs are sequestered within the peritoneal space, they eventually are cleared into the systemic circulation. For this reason, the safe doses of most drugs instilled into the peritoneal cavity are identical to the intravenous dose. The exceptions are drugs with hepatic metabolism such as 5-fluorouracil and gemcitabine. An increased dose of approximately 50% is usually possible with 5-fluorouracil. The dose for a 5-day course of intravenous 5-fluorouracil is approximately 500 mg/m2; for intraperitoneal 5-fluorouracil, the dose is 750 mg/m2 per day. This considerable (50%) increase in the dose of 5-fluorouracil is of great advantage in treating peritoneal carcinomatosis.
Tumor Cell Entrapment
Sugarbaker and colleagues have advanced the "tumor cell entrapment" hypothesis to explain the rapid progression of peritoneal surface malignancy in patients who undergo treatment using surgery alone. This theory relates the high incidence and rapid progression of peritoneal surface implantation to:
Free intraperitoneal tumor emboli as a result of serosal penetration by cancer;
Leakage of malignant cells from transected lymphatics;
Dissemination of malignant cells directly from the cancer specimen as a result of surgical trauma and backflow of venous blood;
Fibrin entrapment of intraabdominal tumor emboli on traumatized peritoneal surfaces;
Progression of these entrapped tumor cells through growth factors involved in the wound healing process.
This phenomenon may cause a high incidence of surgical treatment failure in patients treated for primary gastrointestinal cancer. Also, the reimplantation of malignant cells into peritonectomized surfaces in a reoperative setting must be expected unless intraperitoneal chemotherapy is used.
Chemotherapy employed in the perioperative period not only directly destroys tumor cells, but also eliminates viable platelets, white blood cells and monocytes from the perioneal cavity. This diminishes the promotion of tumor growth associated with the wound healing process. Consequently, the results from use of intraperitoneal chemotherapy show a reduction in local recurrence and peritoneal surface recurrence in patients with intraabdominal cancer. Removal of the leukocytes and monocytes also decreases the ability of the abdomen to resist an infectious process. For this reason, strict aseptic technique is imperative when administering the chemotherapy or handling abdominal tubes and drains.
In order to interrupt this widespread implantation of tumor cells on abdominal and pelvic surfaces, the abdominal cavity is flooded with chemotherapy in a large volume of fluid during the operation (heated intraoperative intraperitoneal chemotherapy) and in the postoperative period (early postoperative intraperitoneal chemotherapy).
Prior Limited Benefits with Intraperitoneal Chemotherapy
The use of intraperitoneal chemotherapy in the past has met with limited success and acceptance by oncologists. There have been three major impediments to greater success. Intracavitary instillation allows very limited penetration of drug into tumor nodules. Only the outermost layer (approximately 1 mm) of a cancer nodule is penetrated by the chemotherapy. This means that only minute tumor nodules can be definitely treated. In most trials, oncologists have attempted to treat established disease, and this selection of patients has caused disappointment with intraperitoneal drug use. Microscopic residual disease is the ideal target for intraperitoneal chemotherapy protocols.
A second cause for limited success with intraperitoneal chemotherapy is a non-uniform drug distribution. A majority of patients treated by drug instillation into the abdomen or pelvis have had prior surgery which invariably causes scarring between peritoneal surfaces. The adhesions create multiple barriers to the free access of fluid. Although the instillation of a large volume of fluid will partially overcome the problems created by adhesions, frequently large surface areas will have no access to chemotherapy. Limited access from adhesions is impossible to predict and may increase with repeated instillations of chemotherapy.
Non-uniform drug distribution after surgery may result from fibrin entrapment. Surgery causes fibrin deposits on surfaces that have been traumatized by the cancer resection. Free intraperitoneal cancer cells become trapped within the fibrin. The fibrin is infiltrated by platelets, neutrophils and monocytes as part of the wound healing process. As collagen is laid down, the tumor cells are entrapped within scar tissue. The scar tissue is dense and poorly penetrated by intraperitoneal chemotherapy.
Non-uniform drug distribution may be caused by gravity. Intraperitoneal fluid does not uniformly distribute itself to anterior and posterior peritoneal surfaces. Gravity pulls the fluid to dependent portions of the abdomen and pelvis; especially the pelvis, paracolic gutters and the right retrohepatic space. Unless the patient actively pursues frequent changes in position, the surfaces between bowel loops and the anterior abdominal wall will remain relatively untreated.
A final obstacle to success with the administration of intraperitoneal chemotherapy encountered in the past is the difficulty and dangers of long-term peritoneal access. There has been no catheter or technical solution to the requirement for reliable repeated access to the peritoneal space. Repeated instillations of large volumes of chemotherapy solution causes great inconvenience and can result in a large number of serious complications. Whether the oncologist chooses repeated paracentesis or an indwelling catheter, complications such as pain upon instillation, bowel perforation, instillation into soft tissues or inability to infuse or drain occur repeatedly. At this time, prolonged peritoneal access is a technical challenge without a known solution.
The problems with prolonged peritoneal access have led some surgical oncologists to adopt what has been referred to as the "big bang" approach. All visible abdominal or pelvic cancer should be completely extirpated by surgery. Then in the operating room, a high dose of heated chemotherapy is delivered to eradicate tiny tumor nodules and microscopic cancer cells that remain. This means that all abdominal and pelvic components of the cancer, including persistent peritoneal surface malignancy, are eliminated. Systemic components of the disease now become the responsibility of the medical oncologist.
Clinical Evidence that Cytoreductive Surgery and Intraperitoneal Chemotherapy is of Benefit to Patients with Peritoneal Surface Malignancy
Treatments for peritoneal carcinomatosis and sarcomatosis have been shown to provide prolonged survival with some patients alive at five years and considered cured. The strategy for treating these patients has always involved three essential components. The first essential component is a complete cytoreduction, utilizing peritonectomy procedures with an attempt to remove all visible tumor. Assuming that microscopic residual disease will eventuate in recurrence in all these patients, the second essential component involves perioperative intraperitoneal chemotherapy. It is becoming increasingly clear that proper patient selection is the third essential component of these treatment strategies. Although no one questions the essential need for complete cytoreduction and for accurate patient selection, many oncologists are not convinced that the intraperitoneal chemotherapy is of benefit to prevent recurrence of peritoneal surface disease. There is data from prospective trials and from clinical observations that suggest that intraperitoneal chemotherapy can reduce or eliminate the recurrence of peritoneal carcinomatosis after surgery.
The first data comes from prospective clinical trials. Sugarbaker and co-workers conducted a trial in patients with poor prognosis colon cancer.(9) Intravenous 5-fluorouracil was randomized against intraperitoneal 5-fluorouracil. Each cycle of treatment was given for 5 days, and the treatments were repeated on a monthly basis for one year. Patients who recurred were explored, and the status of their disease was assessed during the surgery. A statistically significant decrease in the incidence of peritoneal carcinomatosis occurred in patients who had received intraperitoneal 5-fluorouracil (p=0.003). In this small group of poor prognosis patients, there was no improvement in survival but there was a great reduction in the incidence of recurrence of peritoneal carcinomatosis in the patients receiving intraperitoneal 5-fluorouracil .
Several prospective randomized studies in peritoneal carcinomatosis from gastric cancer have been reported. The meta-analysis of eight trials is shown in Figure 18. In all but one of these trials the intraperitoneal chemotherapy was given in the perioperative period. There was an improved survival in the seven trials utilizing perioperative intraperitoneal chemotherapy.(10)
Odds Ratio
Analysis of Studies Using Intraperitoneal Chemotherapy |
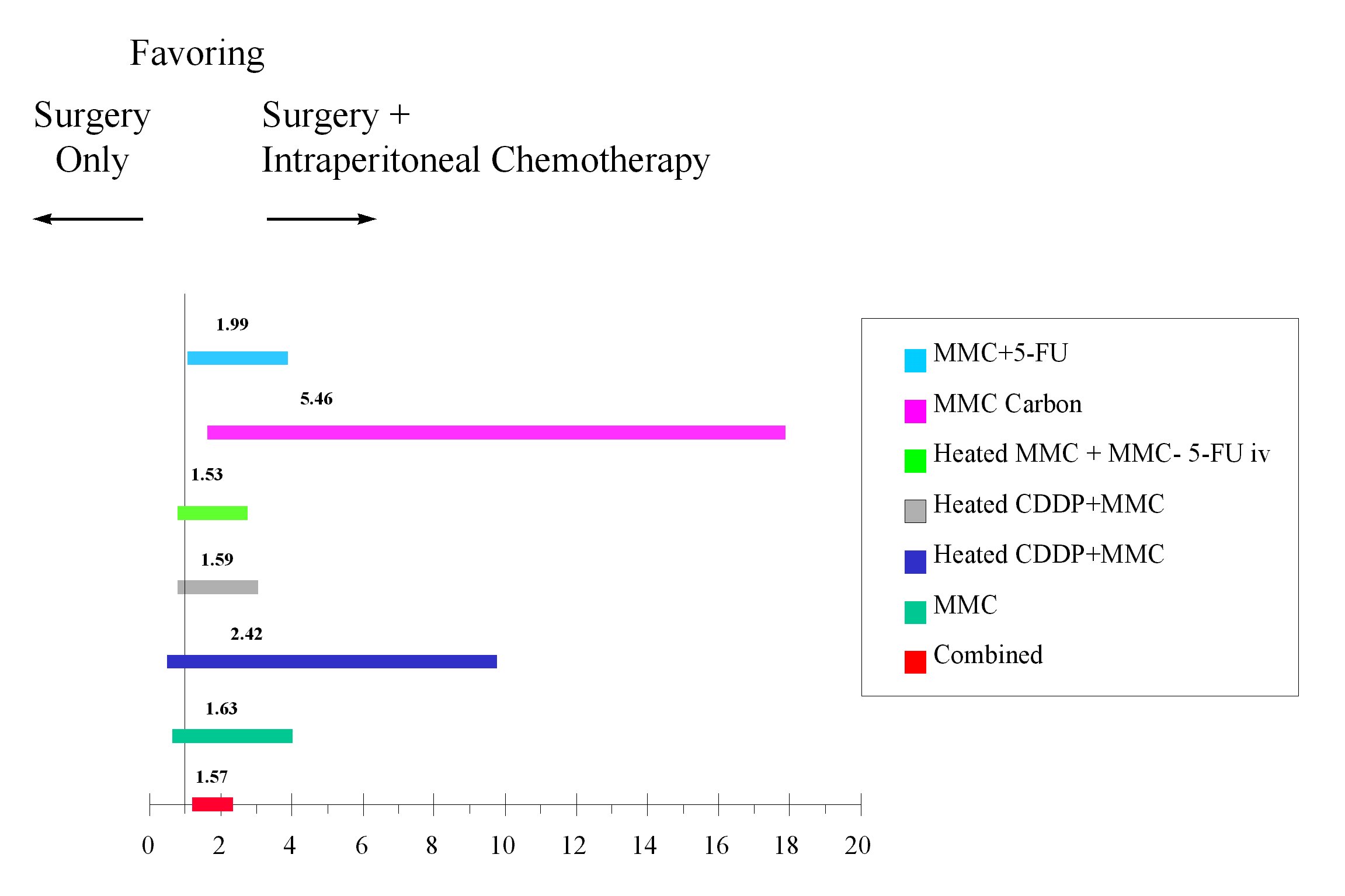 |
Figure 18 |
In the study by Yu et al, perioperative intraperitoneal mitomycin C and 5-fluorouracil were used for the first 5 days following gastrectomy. An analysis by the Cox proportional hazards model showed that the risk of recurrence was nearly twice as great in patients who had surgery alone as compared to those patients who had surgery plus perioperative chemotherapy. In patients with stage III disease, the odds ratio was 4 in favor of the intraperitoneal chemotherapy. In patients who had positive lymph nodes, the odds ratio was 8. This data strongly suggest that microscopic residual disease can be eliminated by adjuvant perioperative intraperitoneal chemotherapy.(11)
Data provided by Gough and co-workers at the Mayo Clinic in patients with pseudomyxoma peritonei show that intraperitoneal chemotherapy is effective in this patient population. In their report on 56 patients, the only long-term survivors were those who had both surgery and intraperitoneal chemotherapy. Patients who had only repeated surgeries had a median survival of 3 years, and only 5% of these patients were alive at the end of 5 years.(12)
The patterns of recurrence in patients treated with intraperitoneal chemotherapy show marked differences in the incidence of recurrence in the abdomen as compared to other anatomic sites. Zoetmulder and colleagues showed that diaphragm perforation in patients with pseudomyxoma peritonei that occurred at the time of cytoreduction was associated with disease progression within the pleural space in 10 of 11 patients.(13) Disease control within the abdomen where intraperitoneal chemotherapy was used occurred in these patients. Small volume disease that entered the chest through a diaphragm perforation without intraperitoneal chemotherapy resulted in progression. A much larger residual disease found in the abdomen was controlled with perioperative intraperitoneal chemotherapy. Likewise, if drain tracts are used in patients with known peritoneal carcinomatosis, they will become involved by disease. Drain tracts in patients undergoing cytoreductive surgery with intraperitoneal chemotherapy seldom, if ever, develop abdominal wall recurrence. Likewise, the abdominal incision is frequently involved if surgery only is used to treat peritoneal carcinomatosis or sarcomatosis. If the surgery is combined with intraperitoneal chemotherapy, disease within the abdominal incision is not seen. This is also true in ovarian cancer patients with vaginal cuff recurrence. If the vaginal cuff is closed without intraperitoneal chemotherapy, ovarian cancer is inoculated into this anatomic site. If intraperitoneal chemotherapy is used after an ovarian cancer cytoreduction, no recurrence within the vaginal cuff has been observed.
A final site for disease occurrence is the laparoscopy ports after laparoscopy in patients with suspect peritoneal carcinomatosis. Almost invariably, the laparoscopy ports become involved by cancer because disease is disseminated by the puncture sites through the abdominal wall.(14)
There is a very definite correlation of the pattern of failure in patients receiving early postoperative intraperitoneal chemotherapy with dye studies that show non-uniform distribution of intraperitoneal chemotherapy in a closed abdomen. Zoetmulder found that recurrence in patients with pseudomyxoma peritonei was most likely to occur within the abdominal incision, within colorectal or gastrojejunal suture lines, and at the base of the small bowel mesentery. (13) In patients given early postoperative intraperitoneal chemotherapy, the closure of the abdominal incision and suture lines prevents adequate chemotherapy access at these sites. Another area for frequent recurrence was the anterior surface of the stomach. Dye studies have shown that the left lobe of the liver almost invariably becomes adherent to the anterior surface of the stomach in patients treated with a closed intraperitoneal chemotherapy technique. Also, dye studies have demonstrated poor chemotherapy access to the base of the small bowel mesentery. This data strongly suggests that pseudomyxoma peritonei recurs where there is imperfect exposure to the intraperitoneal chemotherapy. Other sites that have been noted to have a high incidence of recurrence after use of intraperitoneal chemotherapy are the inverted appendiceal stump and umbilical fissure.
Elias and co-workers reported an interesting pattern of recurrence in those patients who were treated using a peritoneal expander to deliver intraperitoneal chemotherapy. They reported a high incidence of recurrent disease where the peritoneal expander contacted the peritoneal surface at the edges of the abdominal incision. Cancer cells were pressed into the peritoneal surface at this site and were prevented from coming into contact with the chemotherapy and heated fluid (personal communication).
Finally, it should be mentioned that surgery has been used for many decades in an attempt to treat patients with recurrent intraabdominal cancer. The fact that surgery alone has been unsuccessful is well established. Patients with peritoneal seeding have never experienced long-term survival by surgery alone. Data presented in this review clearly shows that there is a high salvage rate in properly selected patients who are treated with cytoreductive surgery combined with adequate intraperitoneal chemotherapy. These data taken together strongly suggest that intraperitoneal chemotherapy is an essential component of treatment protocols for peritoneal surface malignancies.
C. Patient Selection for Treatment
The greatest impediment to lasting benefits from intraperitoneal chemotherapy should be attributed to improper patient selection. A great number of patients with advanced intraabdominal disease have been treated with minimal benefit. Even with extensive cytoreductive surgery and aggressive intraperitoneal chemotherapy, the patient is not likely to have a lasting benefit. Rapid recurrence of intraperitoneal cancer combined with progression of lymph nodal or systemic disease, are likely to interfere with long-term survival in these patients. Patients that benefit must have minimal residual disease isolated to peritoneal surfaces that have access to chemotherapy so that complete eradication of disease can occur. Partial responses are not of great benefit in peritoneal surface malignancies. Complete and durable responses are the reasonable goal. In the natural history of this disease, the time of the initiation of treatment has a great bearing on the benefits achieved. Asymptomatic patients with small volume peritoneal surface malignancy must be selected for intraperitoneal chemotherapy protocols.
Clinical Assessments of Peritoneal Surface Malignancy
In the past, peritoneal carcinomatosis was considered to be a fatal disease process. The only assessment used was either carcinomatosis present with a presumed fatal outcome or carcinomatosis absent with curative treatment options available. Currently, there are four important clinical assessments of peritoneal surface malignancy that need to be used to select patients who will benefit from treatment protocols. These are: 1) The histopathology to assess the invasive character of the malignancy; 2)The preoperative CT scan of abdomen and pelvis; 3) The Peritoneal Cancer Index; 4) The completeness of cytoreduction score.
Histopathology to Assess Invasive Character
The biological aggressiveness of a peritoneal surface malignancy will have profound influence on its treatment options. Non-invasive tumors may have extensive spread on peritoneal surfaces and yet be completely resectable by peritonectomy procedures. Also, these non-invasive malignancies are extremely unlikely to metastasize by lymphatics to lymph nodes and by the blood to liver and other systemic sites. Therefore, protocols for cytoreductive surgery and intraperitoneal chemotherapy may have a curative intent in patients with a large mass of widely disseminated pseudomyxoma peritonei and peritoneal mesothelioma. (15) Also, some low grade sarcomas despite extensive disease progression may be aggressively treated with cure as a goal using cytoreductive surgery and intraperitoneal chemotherapy. Pathology review and an assessment of the invasive or non-aggressive nature of a malignancy is essential to treatment planning.
Preoperative CT Scan
The preoperative CT scan of chest, abdomen and pelvis may be of great value in planning treatments for peritoneal surface malignancy. Systemic metastases can be clinically excluded and pleural surface spread ruled out. Unfortunately, the CT scan should be regarded as an inaccurate test by which to quantitate intestinal type of peritoneal carcinomatosis from adenocarcinoma. The malignant tissue progresses on the peritoneal surfaces and its shape conforms to the normal contours of the abdominopelvic structures. This is quite different from the metastatic process in the liver or lung which progresses as three-dimensional tumor nodules and can be accurately assessed by CT. (16)
However, the CT scan has been of great help in locating and quantitating mucinous adenocarcinoma within the peritoneal cavity. (17) These tumors produce copious colloid material that is readily distinguished by shape and by density from normal structures. Using two distinctive radiologic criteria, those patients with resectable mucinous peritoneal carcinomatosis can be selected from those with non-resectable malignancy. This keeps patients who are unlikely to benefit from reoperative surgery from undergoing cytoreductive surgical procedures. The two radiologic criteria found to be most useful are:
- Segmental obstruction of small bowel.
- Presence of tumor nodules greater than 5 cm in diameter on small bowel surfaces or directly adjacent to small bowel mesentery.
These criteria reflect radiologically the biology of the mucinous adenocarcinoma. Obstructed segments of bowel signal an invasive character of malignancy on small bowel surfaces that would be unlikely to be completely cytoreduced. Mucinous cancer on small bowel or small bowel mesentery indicates that the mucinous cancer is no longer redistributed. This means that small bowel surfaces or small bowel mesentery will have residual disease after cytoreduction, because these surfaces are impossible to peritonectomize (Figure 19 and Figure 20).
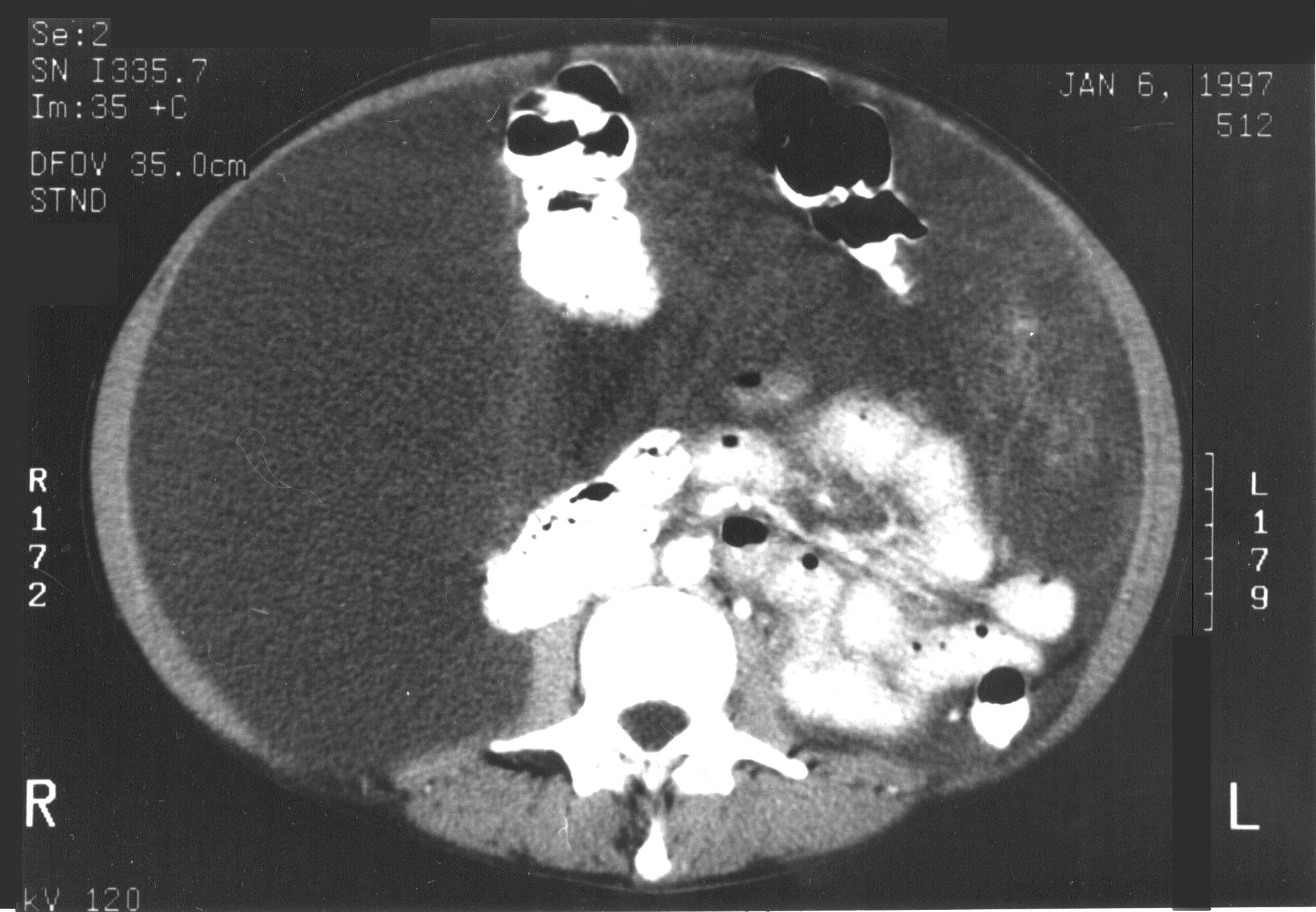 |
Figure 19 |
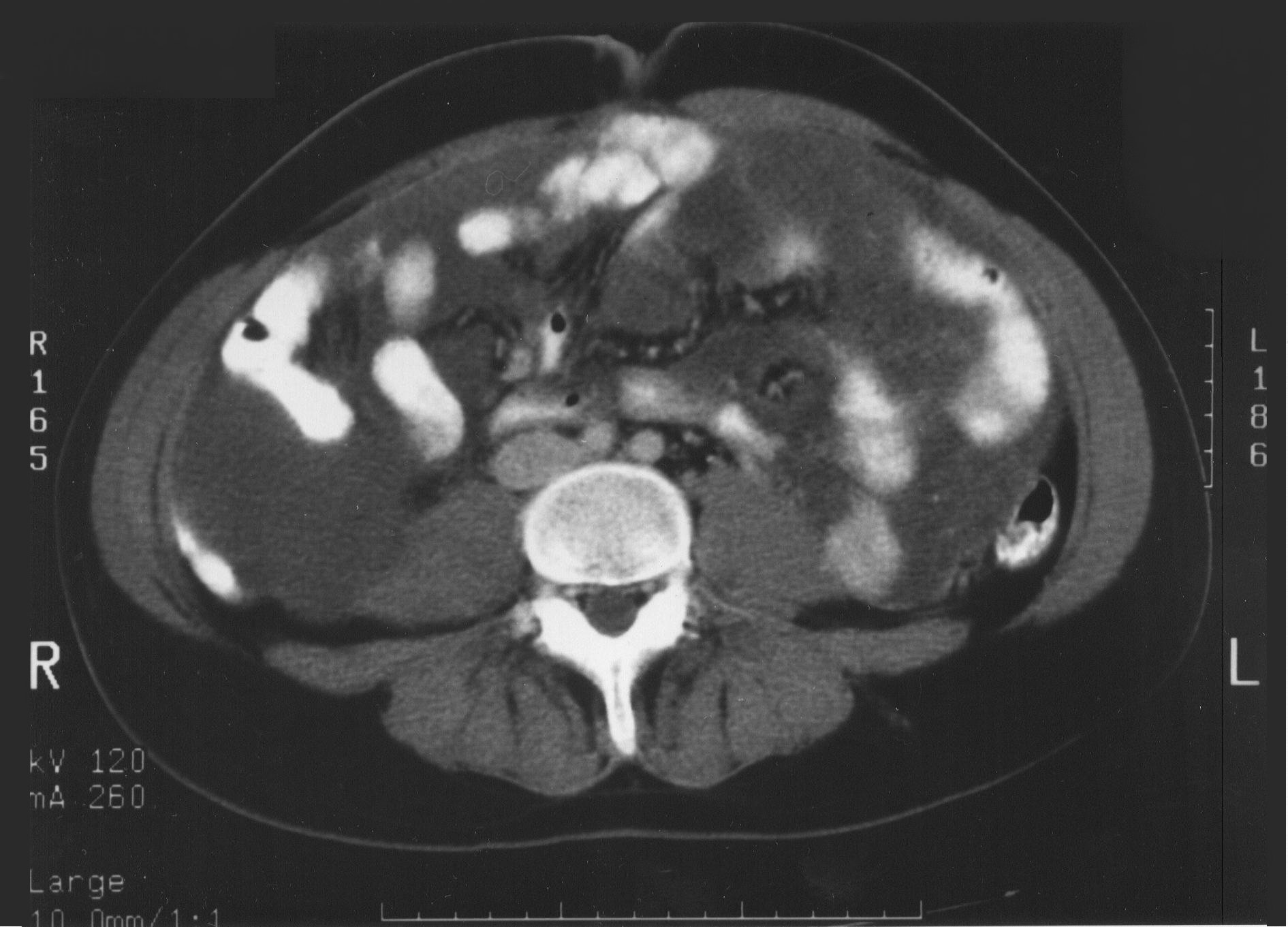 |
Figure 20 |
The CT is also of great help in the identification of nodules of recurrent sarcoma and sarcomatosis. The recurrences on peritoneal surfaces are nodular and the result of fibrin entrapment of traumatically disseminated sarcoma cells. In a CT scan with maximal filling of bowel with oral contrast, even small 1 cm nodular sarcoma recurrences are imaged.
Peritoneal Cancer Index
The third assessment of peritoneal surface malignancy is the Peritoneal Cancer Index. This is a clinical integration of both peritoneal implant size and distribution of peritoneal surface malignancy (Figure 21). It should be used in the decision making process as the abdomen is explored. To arrive at a score, the size of intraperitoneal nodules must be assessed. The lesion size or LS score should be used. An LS-0 score means that no malignant deposits are visualized. An LS-1 score signifies tumor nodules less than 0.5 cm present. The number of nodules is not scored, only the size of the largest nodules. An LS-2 score signifies tumor nodules between 0.5 and 5.0 cm present. LS-3 signifies tumor nodules greater than 5.0 cm in any dimension present. If there is a confluence of tumor, the lesion size is scored as 3.
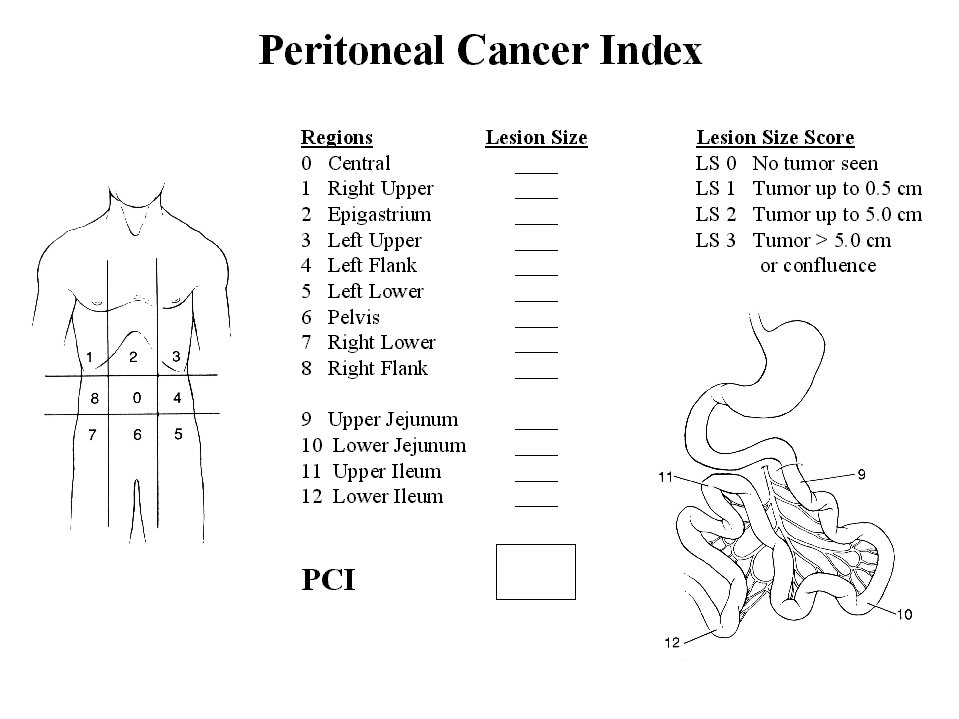 |
Figure 21 |
In order to assess the distribution of peritoneal surface disease, the abdominopelvic regions are utilized. For each of these 13 regions, a lesion size score is determined. The summation of the lesion size score in each of the 13 abdominopelvic regions is the Peritoneal Cancer Index for that patient. A maximal score is 39 (13 x 3).
The Peritoneal Cancer Index has been validated to date in 3 separate situations. First, Steller and colleagues used it successfully to quantitate intraperitoneal tumor in a murine peritoneal carcinomatosis model.(18) Gomez and co-workers showed that the Peritoneal Cancer Index could be used to predict long-term survival in patients with peritoneal carcinomatosis from colon cancer having a second cytoreduction.(19) Berthet and co-workers showed that the Peritoneal Cancer Index predicted benefits for treatment of peritoneal sarcomatosis from recurrent visceral or parietal sarcoma.(20) In both clinical studies, the patients with a favorable prognosis had a score of less than 12.
There are some caveats in the use of the Peritoneal Cancer Index. First, non-invasive malignancy on peritoneal surfaces may be completely cytoreduced. Diseases such as pseudomxyoma peritonei and peritoneal mesothelioma are in this category. With these "benign tumors," the status of the abdomen and pelvis after cytoreduction may have no relationship to its status at the time of abdominal exploration. In other words, even though the surgeons may find an abdomen with a Peritoneal Cancer Index of 39, it can be converted to an index of 0 by cytoreduction. In these diseases, the prognosis will only be related to the condition of the abdomen after the cytoreduction (completeness of cytoreduction score).
A second caveat for the Peritoneal Cancer Index is invasive cancer at crucial anatomic sites. For example, invasive cancer not cleanly resected on the common bile duct will cause a poor prognosis despite a low Peritoneal Cancer Index. Invasion of the base of the bladder or unresectable disease on a pelvic side wall may, by itself, result in residual invasive cancer after cytoreduction and imply a poor prognosis. Also, unresectable cancer at numerous sites on the small bowel may by itself confer a poor prognosis. In other words, invasive cancer at crucial anatomic sites may function as systemic disease in assessing prognosis with invasive cancer. Since long-term survival can only occur in patients with a complete cytoreduction, residual disease at anatomically crucial sites may override a favorable score with the Peritoneal Cancer Index.
Completeness of Cytoreduction Score
The final assessment to be used to assess prognosis with peritoneal surface malignancy is the completeness of cytoreduction (CC) score. This information is of less value to the surgeon in planning treatments than the Peritoneal Cancer Index. The CC score is not available until after the cytoreduction is complete, rather than as the abdomen is being explored. If during exploration it becomes obvious that cytoreduction will not be complete, the surgeon may decide that a palliative debulking that will provide symptomatic relief is appropriate and discontinue plans for an aggressive cytoreduction with intraperitoneal chemotherapy. In both non-invasive and invasive peritoneal surface malignancy, the completeness of cytoreduction score is a major prognostic indicator. It has been shown to function with accuracy in pseudomyxoma peritonei, colon cancer with peritoneal carcinomatosis and sarcomatosis.(15, 20, 21)
The size of peritoneal implants used to determine the CC score may vary with the primary site of the peritoneal carcinomatosis. More chemotherapy responsive malignancies, such as ovarian cancer, may be eradicated even though larger sized tumor nodules remain after cytoreduction. For gastrointestinal cancer the completeness of cytoreduction score has been defined as follows: A CC-0 score indicates that no peritoneal seeding was exposed during the complete exploration. A CC-1 score indicates that tumor nodules persisting after cytoreduction are less than 2.5mm. This is a nodule size thought to be penetrable by intracavity chemotherapy and would, therefore, be designated a complete cytoreduction. A CC-2 score indicates tumor nodules between 2.5mm and 2.5cm. A CC-3 score indicates tumor nodules greater than 2.5cm or a confluence of unresectable tumor nodules at any site within the abdomen or pelvis. CC-2 and CC-3 cytoreduction are considered incomplete.
I. Introduction II.Principles of Management III. Current
Methodologies for Delivery of Intraperitoneal Chemotherapy
IV. Clinical
Results of Treatment V. Ethical Considerations
in Clinical Studies with Peritoneal Surface Malignancy VI.
References